|
Nitro Ligand
In organometallic chemistry, transition metal complexes of nitrite describes families of coordination complexes containing one or more nitrite () ligands. Although the synthetic derivatives are only of scholarly interest, metal-nitrite complexes occur in several enzymes that participate in the nitrogen cycle. Structure and bonding Bonding modes Three linkage isomers are common for nitrite ligands, O-bonded, N-bonded, and bidentate O,O-bonded. The former two isomers have been characterized for the pentamminecobalt(III) system, i.e. and , referred to as N-nitrito and O-nitrito, respectively. These two forms are sometimes called nitro and nitrito. These isomers can be interconverted in some complexes. An example of chelating nitrite is – "bipy" is the bidentate ligand 2,2′-bipyridyl. This bonding mode is sometimes described as κ2O,O-.. The kinetically-favored O-bonded isomer converts to . In its reaction with ferric porphyrin complexes, nitrite gives the O-bonded isomer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
X-ray Crystallography
X-ray crystallography is the experimental science of determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to Diffraction, diffract in specific directions. By measuring the angles and intensities of the X-ray diffraction, a crystallography, crystallographer can produce a three-dimensional picture of the density of electrons within the crystal and the positions of the atoms, as well as their chemical bonds, crystallographic disorder, and other information. X-ray crystallography has been fundamental in the development of many scientific fields. In its first decades of use, this method determined the size of atoms, the lengths and types of chemical bonds, and the atomic-scale differences between various materials, especially minerals and alloys. The method has also revealed the structure and function of many biological molecules, including vitamins, drugs, proteins and nucleic acids such as DNA. X-ray crystall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ligands
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs, often through Lewis bases. The nature of metal–ligand bonding can range from covalent to ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acidic "ligands". Metals and metalloids are bound to ligands in almost all circumstances, although gaseous "naked" metal ions can be generated in a high vacuum. Ligands in a complex dictate the reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand selection requires critical consideration in many practical areas, including bioinorganic and medicinal chemistry, homogeneous catalysis, and environme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isonitrosyl
Sodium nitroprusside, a medicinally significant metal nitrosyl-pentacyanoferrate (Fe-III) compound, used to treat complexes that contain nitric oxide">hypertension. Metal nitrosyl complexes are complex (chemistry)">complexes that contain nitric oxide, NO, bonded to a transition metal. Many kinds of nitrosyl complexes are known, which vary both in structure and coligand. Bonding and structure Most complexes containing the NO ligand can be viewed as derivatives of the nitrosyl cation, NO+. The nitrosyl cation is isoelectronic with carbon monoxide, thus the bonding between a nitrosyl ligand and a metal follows the same principles as the bonding in carbonyl complexes. The nitrosyl cation serves as a two-electron donor to the metal and accepts electrons from the metal via back-bonding. The compounds Co(NO)(CO)3 and Ni(CO)4 illustrate the analogy between NO+ and CO. In an electron-counting sense, two linear NO ligands are equivalent to three CO groups. This trend is illustrate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrite Reductase
Nitrite reductase refers to any of several classes of enzymes that catalyze the reduction of nitrite. There are two classes of NIR's. A multi haem enzyme reduces NO2− to a variety of products. Copper containing enzymes carry out a single electron transfer to produce nitric oxide. Iron based There are several types of iron based enzymes. Cytochrome cd1, or ''Pseudomonas'' cytochrome oxidase contains two c and two d type hemes with two polypeptide chains. Different forms of this reductase catalyze the formation of nitric oxide or nitrous oxide. A version of this compound was originally called errocytochrome c-551:oxidoreductase It was initially considered an oxidase. It catalyzes the reduction of NO2− to NO. This tetraheme enzyme has two Protein subunit">subunits, each containing a c-type and a d-type heme. The reduced d hemes bind nitrite and convert it to product. Cytochrome c nitrite reductase (ccNIR) is a multiheme enzyme that converts nitrite to ammonia on each ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heme
Heme (American English), or haem (Commonwealth English, both pronounced /Help:IPA/English, hi:m/ ), is a ring-shaped iron-containing molecule that commonly serves as a Ligand (biochemistry), ligand of various proteins, more notably as a Prosthetic group, component of hemoglobin, which is necessary to bind oxygen in the bloodstream. It is composed of four pyrrole rings with 2 Vinyl group, vinyl and 2 propionic acid side chains. Heme is biosynthesized in both the bone marrow and the liver. Heme plays a critical role in multiple different redox reactions in mammals, due to its ability to carry the oxygen molecule. Reactions include oxidative metabolism (cytochrome c oxidase, succinate dehydrogenase), xenobiotic detoxification via cytochrome P450 pathways (including Drug metabolism, metabolism of some drugs), gas sensing (Guanylate cyclase, guanyl cyclases, nitric oxide synthase), and microRNA processing (DGCR8). Heme is a coordination complex "consisting of an iron ion coordinated ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrite Oxidoreductase
Nitrite oxidoreductase (NOR or NXR) is an enzyme involved in nitrification. It is the last step in the process of aerobic ammonia oxidation, which is carried out by two groups of nitrifying bacteria: ammonia oxidizers such as '' Nitrosospira'', '' Nitrosomonas,'' and '' Nitrosococcus'' convert ammonia to nitrite, while nitrite oxidizers such as '' Nitrobacter'' and '' Nitrospira'' oxidize nitrite to nitrate. NXR is responsible for producing almost all nitrate found in nature. NXR belongs to the class of EC numbers 1.7.2- where 1 describes an oxidoreductase, 1.7 describes nitrogen compounds as donors, and 1.7.2- describes cytochromes as acceptors. Structure NXR is composed of 2 mainly known subunits; nitrite oxidoreductase α (NxrA), and nitrite oxidoreductase β (NxrB) (sometimes written as NorA and NorB). However, recent studies describe a third and fourth subunit, NxrC and NxrT The enzyme's known active site is on the NxrA subunit. There are two types of NXR; one where the N ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrate
Nitrate is a polyatomic ion with the chemical formula . salt (chemistry), Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are solubility, soluble in water. An example of an insoluble nitrate is bismuth oxynitrate. Chemical structure The nitrate anion is the conjugate acid, conjugate base of nitric acid, consisting of one central nitrogen atom surrounded by three identically bonded oxygen atoms in a trigonal planar arrangement. The nitrate ion carries a formal charge of −1. This charge results from a combination formal charge in which each of the three oxygens carries a − charge, whereas the nitrogen carries a +1 charge, all these adding up to formal charge of the polyatomic nitrate ion. This arrangement is commonly used as an example of Resonance (chemistry), resonance. Like the isoelectronic carbonate ion, the nitrate ion can be represented by three resonance structures: Che ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pungent smell. It is widely used in fertilizers, refrigerants, explosives, cleaning agents, and is a precursor for numeous chemicals. Biologically, it is a common nitrogenous waste, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to fertilisers. Around 70% of ammonia produced industrially is used to make fertilisers in various forms and composition, such as urea and diammonium phosphate. Ammonia in pure form is also applied directly into the soil. Ammonia, either directly or indirectly, is also a building block for the synthesis of many chemicals. In many countries, it is classified as an List of extremely hazardous substances, extremely hazardous substance. Ammonia is toxic, cau ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrosyl Complex
Sodium nitroprusside, a medicinally significant metal nitrosyl-pentacyanoferrate (Fe-III) compound, used to treat complexes that contain nitric oxide">hypertension. Metal nitrosyl complexes are complex (chemistry)">complexes that contain nitric oxide, NO, bonded to a transition metal. Many kinds of nitrosyl complexes are known, which vary both in structure and coligand. Bonding and structure Most complexes containing the NO ligand can be viewed as derivatives of the nitrosyl cation, NO+. The nitrosyl cation is isoelectronic with carbon monoxide, thus the bonding between a nitrosyl ligand and a metal follows the same principles as the bonding in carbonyl complexes. The nitrosyl cation serves as a two-electron donor to the metal and accepts electrons from the metal via back-bonding. The compounds Co(NO)(CO)3 and Ni(CO)4 illustrate the analogy between NO+ and CO. In an electron-counting sense, two linear NO ligands are equivalent to three CO groups. This trend is illustrate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
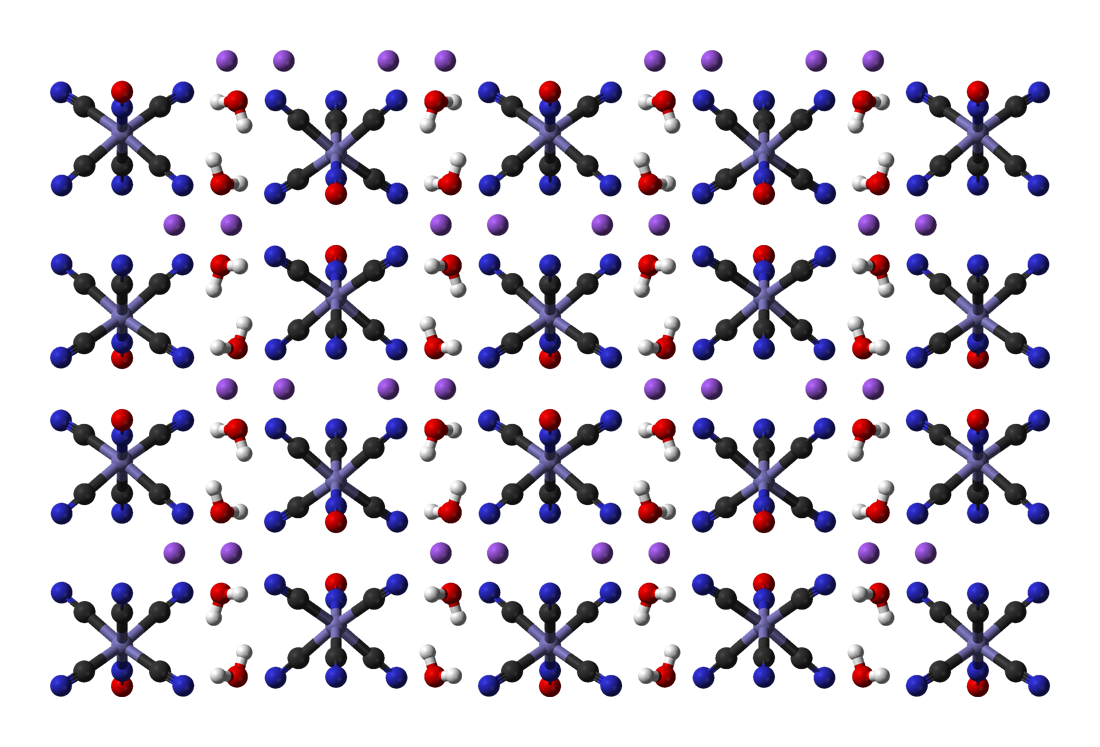
Nitroprusside
Sodium nitroprusside (SNP), sold under the brand name Nitropress among others, is a medication used to lower blood pressure. This may be done if the blood pressure is very high and resulting in symptoms, in certain types of heart failure, and during surgery to decrease bleeding. It is used by continuous injection into a vein. Onset is nearly immediate and effects last for up to ten minutes. It is available as a generic medication. Side effects and mechanism Common side effects include low blood pressure and cyanide toxicity. Other serious side effects include methemoglobinemia. It is not generally recommended during pregnancy due to concerns of side effects. High doses are not recommended for more than ten minutes. It works by increasing nitric oxide levels in the blood, which increases cGMP levels in cells, and causes dilation of blood vessels. History, society and culture Sodium nitroprusside was discovered as early as 1850 and found to be useful in medicine in 1928. It ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |