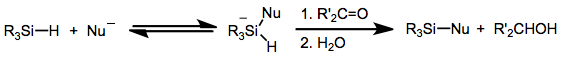|
Nickel Bis(cyclooctadiene)
Bis(cyclooctadiene)nickel(0) is the organonickel compound with the formula Ni(C8H12)2, also written Ni(cod)2. It is a diamagnetic coordination complex featuring tetrahedral nickel(0) bound to the alkene groups in two 1,5-cyclooctadiene ligands. This highly air-sensitive yellow solid is a common source of Ni(0) in chemical synthesis. Preparation and properties The complex is prepared by reduction of anhydrous nickel(II) acetylacetonate in the presence of the diolefin: :Ni(acac)2 + 2 cod + 2 AlEt3 → Ni(cod)2 + 2 acacAlEt2 + C2H6 + C2H4 Ni(cod)2 is moderately soluble in several organic solvents. If exposed to air, the solid oxidizes in a few minutes to nickel(II) oxide. As a result, this compound is generally handled in a glovebox. Reactions The reactivity of Ni(cod)2 has been extensively examined. One or both 1,5-cyclooctadiene ligands are readily displaced by phosphines, phosphites, bipyridine, and isocyanides. Oxidation gives the highly reactive monocation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of petroleum and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon. Benzene is a colorless and highly Combustibility and flammability, flammable liquid with a sweet smell, and is partially responsible for the aroma of gasoline. It is used primarily as a Precursor (chemistry), precursor to the manufacture of chemicals with more complex structures, such as ethylbenzene and cumene, of which billions of kilograms are produced annually. Although benzene is a major Chemical industry, industrial che ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Syntheses
''Inorganic Syntheses'' is a book series which aims to publish "detailed and foolproof" procedures for the synthesis of inorganic compounds. Although this series of books are edited, they usually are referenced like a journal, without mentioning the names of the checkers (referees) or the editor. A similar format is usually followed for the series '' Organic Syntheses''. Volumes See also * Organic SynthesesReferences {{chem-book-stub Book series introduced in 1939 ...[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrosilane
Hydrosilanes are tetravalent silicon compounds containing one or more Si-H bond. The parent hydrosilane is silane (SiH4). Commonly, hydrosilane refers to organosilicon derivatives. Examples include phenylsilane (PhSiH3) and triethoxysilane ((C2H5O)3SiH). Polymers and oligomers terminated with hydrosilanes are resins that are used to make useful materials like caulks. Synthesis Trichlorosilane is produced commercially by the reaction of hydrogen chloride with silicon: :Si + 3 HCl → HSiCl3 + H2 Many alkoxy hydrosilanes are generated by alcoholysis of trichlorosilane. One example is triethoxysilane: :HSiCl3 + 3EtOH → HSi(OEt)3 + 3 HCl Organohydrosilanes can be prepared by partial hydrosilation of silane itself: :SiH4 + 3 C2H4 → HSi(C2H5)3 In the laboratory, hydrosilanes classically are prepared by treating chlorosilanes with hydride reagents, such as lithium aluminium hydride: :4ClSi(C2H5)3 + LiAlH4 → 4HSi(C2H5)3 + LiAlCl4 Structure The silicon-to-hydrogen bond is long ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anisole
Anisole, or methoxybenzene, is an organic compound with the formula . It is a colorless liquid with a smell reminiscent of anise seed, and in fact many of its derivatives are found in natural and artificial fragrances. The compound is mainly made synthetically and is a precursor to other synthetic compounds. Structurally, it is an ether () with a methyl () and phenyl () group attached. Anisole is a standard reagent of both practical and pedagogical value. Reactivity Anisole undergoes electrophilic aromatic substitution reaction at a faster speed than benzene, which in turn reacts more quickly than nitrobenzene. The methoxy group is an ortho/para directing group, which means that electrophilic substitution preferentially occurs at these three sites. The enhanced nucleophilicity of anisole vs. benzene reflects the influence of the methoxy group, which renders the ring more electron-rich. The methoxy group strongly affects the pi cloud of the ring as a mesomeric electron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Weakly Coordinating Anion
Anions that interact weakly with cations are termed non-coordinating anions, although a more accurate term is weakly coordinating anion. Non-coordinating anions are useful in studying the reactivity of electrophilic cations. They are commonly found as counterions for cationic metal complexes with an unsaturated coordination sphere. These special anions are essential components of homogeneous alkene polymerisation catalysts, where the active catalyst is a coordinatively unsaturated, cationic transition metal complex. For example, they are employed as counterions for the 14 valence electron cations C5H5)2ZrRsup>+ (R = methyl or a growing polyethylene chain). Complexes derived from non-coordinating anions have been used to catalyze hydrogenation, hydrosilylation, oligomerization, and the living polymerization of alkenes. The popularization of non-coordinating anions has contributed to increased understanding of agostic complexes wherein hydrocarbons and hydrogen serve as ligand ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isocyanide
An isocyanide (also called isonitrile or carbylamine) is an organic compound with the functional group –. It is the isomer of the related nitrile (–C≡N), hence the prefix is ''isocyano''.IUPAC Goldboo''isocyanides''/ref> The organic fragment is connected to the isocyanide group through the nitrogen atom, not via the carbon. They are used as building blocks for the synthesis of other compounds. Properties Structure and bonding The C-N distance in isocyanides is 115.8 pm in methyl isocyanide. The C-N-C angles are near 180°. Akin to carbon monoxide, isocyanides are described by two Resonance (chemistry), resonance structures, one with a triple bond between the nitrogen and the carbon and one with a double bond between. The π lone pair of the nitrogen stabilizes the structure and is responsible of the linearity of isocyanides, although the reactivity of isocyanides reflects some carbene character, at least in a formal sense. Thus, both resonance structures are useful repres ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bipyridine
Bipyridines are a family of organic compounds with the formula (C5H4N)2, consisting of two pyridyl (C5H4N) rings. Pyridine is an aromatic nitrogen-containing heterocycle. The bipyridines are all colourless solids, which are soluble in organic solvents and slightly soluble in water. Bipyridines, especially the 4,4' isomer, are mainly of significance in pesticides. Six isomers of bipyridine exist, but two are prominent. 2,2′-bipyridine, also known as bipyridyl, dipyridyl, and dipyridine, is a popular ligand in coordination chemistry 2,2′-Bipyridine 2,2′-Bipyridine (2,2′-bipy) is a chelating ligand that forms complexes with most transition metal ions that are of broad academic interest. Many of these complexes have distinctive optical properties, and some are of interest for analysis. Its complexes are used in studies of electron and energy transfer, supramolecular, and materials chemistry, and catalysis. 2,2′-Bipyridine is used in the manufacture of diquat. 4, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glovebox
A glovebox (or glove box) is a sealed container that is designed to allow one to manipulate objects where a separate atmosphere is desired. Built into the sides of the glovebox are gloves arranged in such a way that the user can place their hands into the gloves and perform tasks inside the box without breaking containment. Part or all of the box is usually transparent to allow the user to see what is being manipulated. A smaller antechamber compartment is used to transport items into or out of the main chamber without compromising the internal environment. Antechambers are much smaller than the main chambers so they can be exposed to ambient conditions more often and achieve inert conditions quickly. Two types of gloveboxes exist. The first allows a person to work with hazardous substances, such as radioactive materials or infectious disease agents, and the second allows manipulation of substances that must be contained within a very high purity inert atmosphere, such as argon o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nickel(II) Oxide
Nickel(II) oxide is the chemical compound with the formula . It is the principal oxide of nickel. It is classified as a basic metal oxide. Several million kilograms are produced annually of varying quality, mainly as an intermediate in the production of nickel alloys. The mineralogical form of , bunsenite, is very rare. Other nickel(III) oxides have been claimed, for example: and , but remain unproven. Production can be prepared by multiple methods. Upon heating above 400 °C, nickel powder reacts with oxygen to give . In some commercial processes, green nickel oxide is made by heating a mixture of nickel powder and water at 1000 °C; the rate for this reaction can be increased by the addition of ."Handbook of Inorganic Chemicals", Pradniak, Pradyot; McGraw-Hill Publications,2002 The simplest and most successful method of preparation is through pyrolysis of nickel(II) compounds such as the hydroxide, nitrate, and carbonate, which yield a light green powder. Synthesi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triethylaluminium
Triethylaluminium is one of the simplest examples of an organoaluminium compound. Despite its name the compound has the formula Al2( C2H5)6 (abbreviated as Al2Et6 or TEA). This colorless liquid is pyrophoric. It is an industrially important compound, closely related to trimethylaluminium. Structure and bonding The structure and bonding in Al2R6 and diborane are analogous (R = alkyl). Referring to Al2Me6, the Al-C(terminal) and Al-C(bridging) distances are 1.97 and 2.14 Å, respectively. The Al center is tetrahedral. The carbon atoms of the bridging ethyl groups are each surrounded by five neighbors: carbon, two hydrogen atoms and two aluminium atoms. The ethyl groups interchange readily intramolecularly. At higher temperatures, the dimer cracks into monomeric AlEt3. Synthesis and reactions Triethylaluminium can be formed via several routes. The discovery of an efficient route was a significant technological achievement. The multistep process uses aluminium, hydrogen gas, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is mainly used as a precursor to polymers. Being polar and having a wide liquid range, THF is a versatile solvent. It is an isomer of another solvent, butanone. Production About 200,000 tonnes of tetrahydrofuran are produced annually. The most widely used industrial process involves the acid-catalyzed dehydration of 1,4-Butanediol, 1,4-butanediol. Ashland Inc., Ashland/ISP is one of the biggest producers of this chemical route. The method is similar to the production of diethyl ether from ethanol. The butanediol is derived from Condensation reaction, condensation of acetylene with formaldehyde followed by hydrogenation. DuPont developed a process for producing THF by oxidizing Butane#Isomers, ''n''-butane to crude maleic anhydride, follow ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nickel(II) Acetylacetonate
Nickel(II) bis(acetylacetonate) is a coordination complex with the formula i(acac)2sub>3, where acac is the anion derived from deprotonation of acetylacetone. It is a dark green paramagnetic solid that is soluble in organic solvents such as toluene. It reacts with water to give the blue-green diaquo complex Ni(acac)2(H2O)2. Structure and properties Anhydrous nickel(II) acetylacetonate exists as molecules of Ni3(acac)6. The three nickel atoms are approximately collinear and each pair of them is bridged by two μ2 oxygen atoms. Each nickel atom has tetragonally distorted octahedral geometry, caused by the difference in the length of the Ni–O bonds between the bridging and non-bridging oxygens. Ni3(acac)6 molecules are almost centrosymmetric, despite the non-centrosymmetric point group of the ''cis''-Ni(acac)2 "monomers," which is uncommon. The trimeric structure allows all nickel centers to achieve an octahedral coordination. The trimer is only formed if intramolecular sharin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |