|
Neuraminidase Ribbon Diagram
Exo-α-sialidase (EC 3.2.1.18, sialidase, neuraminidase; systematic name acetylneuraminyl hydrolase) is a glycoside hydrolase that cleaves the glycosidic linkages of neuraminic acids: : Hydrolysis of α-(2→3)-, α-(2→6)-, α-(2→8)- glycosidic linkages of terminal sialic acid residues in oligosaccharides, glycoproteins, glycolipids, colominic acid and synthetic substrates Neuraminidase enzymes are a large family, found in a range of organisms. The best-known neuraminidase is the viral neuraminidase, a drug target for the prevention of the spread of influenza infection. The viral neuraminidases are frequently used as antigenic determinants found on the surface of the influenza virus. Some variants of the influenza neuraminidase confer more virulence to the virus than others. Other homologues are found in mammalian cells, which have a range of functions. At least four mammalian sialidase homologues have been described in the human genome (see NEU1, NEU2, NEU3, NEU4). Sial ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Swiss-Prot
UniProt is a freely accessible database of protein sequence and functional information, many entries being derived from genome sequencing projects. It contains a large amount of information about the biological function of proteins derived from the research literature. It is maintained by the UniProt consortium, which consists of several European bioinformatics organisations and a foundation from Washington, DC, United States. The UniProt consortium The UniProt consortium comprises the European Bioinformatics Institute (EBI), the Swiss Institute of Bioinformatics (SIB), and the Protein Information Resource (PIR). EBI, located at the Wellcome Trust Genome Campus in Hinxton, UK, hosts a large resource of bioinformatics databases and services. SIB, located in Geneva, Switzerland, maintains the ExPASy (Expert Protein Analysis System) servers that are a central resource for proteomics tools and databases. PIR, hosted by the National Biomedical Research Foundation (NBRF) at the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zanamivir
Zanamivir is a medication used to treat and prevent influenza caused by influenza A and influenza B viruses. It is a neuraminidase inhibitor and was developed by the Australian biotech firm Biota Holdings. It was licensed to Glaxo in 1990 and approved in the US in 1999, only for use as a treatment for influenza. In 2006, it was approved for prevention of influenza A and B. Zanamivir was the first neuraminidase inhibitor commercially developed. It is marketed by GlaxoSmithKline under the trade name Relenza as a powder for oral inhalation. Medical uses Zanamivir is used for the treatment of infections caused by influenza A and influenza B viruses, but in otherwise-healthy individuals, benefits overall appear to be small. It decreases the risk of one's getting symptomatic, but not asymptomatic influenza. The combination of diagnostic uncertainty, the risk for virus strain resistance, possible side effects and financial cost outweigh the small benefits of zanamivir for the prophylax ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oseltamivir
Oseltamivir, sold under the brand name Tamiflu, is an antiviral medication used to treat and prevent influenza A and influenza B, viruses that cause the flu. Many medical organizations recommend it in people who have complications or are at high risk of complications within 48 hours of first symptoms of infection. They recommend it to prevent infection in those at high risk, but not the general population. The Centers for Disease Control and Prevention (CDC) recommends that clinicians use their discretion to treat those at lower risk who present within 48 hours of first symptoms of infection. It is taken by mouth, either as a pill or liquid. Recommendations regarding oseltamivir are controversial as are criticisms of the recommendations. A 2014 Cochrane Review concluded that oseltamivir does not reduce hospitalizations, and that there is no evidence of reduction in complications of influenza. Two meta-analyses have concluded that benefits in those who are otherwise healthy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lectin
Lectins are carbohydrate-binding proteins that are highly specific for sugar groups that are part of other molecules, so cause agglutination of particular cells or precipitation of glycoconjugates and polysaccharides. Lectins have a role in recognition at the cellular and molecular level and play numerous roles in biological recognition phenomena involving cells, carbohydrates, and proteins. Lectins also mediate attachment and binding of bacteria, viruses, and fungi to their intended targets. Lectins are ubiquitous in nature and are found in many foods. Some foods, such as beans and grains, need to be cooked, fermented or sprouted to reduce lectin content. Some lectins are beneficial, such as CLEC11A, which promotes bone growth, while others may be powerful toxins such as ricin. Lectins may be disabled by specific mono- and oligosaccharides, which bind to ingested lectins from grains, legumes, nightshade plants, and dairy; binding can prevent their attachment to the carbohydr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta-propeller
In structural biology, a beta-propeller (β-propeller) is a type of all-β protein architecture characterized by 4 to 8 highly symmetrical blade-shaped beta sheets arranged toroidally around a central axis. Together the beta-sheets form a funnel-like active site. Structure Each beta-sheet typically has four anti-parallel β-strands arranged in the beta-zigzag motif. The strands are twisted so that the first and fourth strands are almost perpendicular to each other. There are five classes of beta-propellers, each arrangement being a highly symmetrical structure with 4–8 beta sheets, all of which generally form a central tunnel that yields pseudo-symmetric axes. While, the protein's official active site for ligand-binding is formed at one end of the central tunnel by loops between individual beta-strands, protein-protein interactions can occur at multiple areas around the domain. Depending on the packing and tilt of the beta-sheets and beta-strands, the beta-propeller may ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hemagglutinin (influenza)
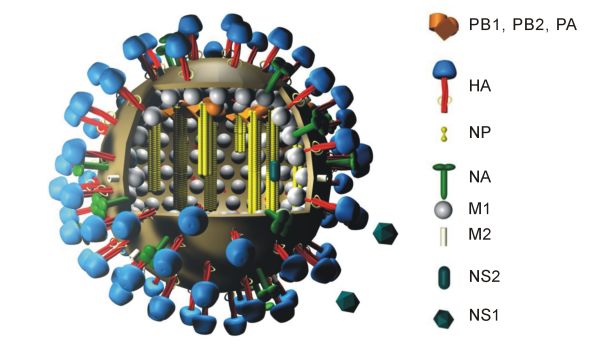
Influenza hemagglutinin (HA) or haemagglutinin ">/sup> (British English) is a homotrimeric glycoprotein found on the surface of influenza viruses and is integral to its infectivity. Hemagglutinin is a Class I Fusion Protein, having multifunctional activity as both an attachment factor and membrane fusion protein. Therefore, HA is responsible for binding Influenza virus to sialic acid on the surface of target cells, such as cells in the upper respiratory tract or erythrocytes, causing as a result the internalization of the virus. Secondarily, HA is responsible for the fusion of the viral envelope with the late endosomal membrane once exposed to low pH (5.0-5.5). The name "hemagglutinin" comes from the protein's ability to cause red blood cells (erythrocytes) to clump together ("agglutinate") ''in vitro''. Subtypes Hemagglutinin (HA) in influenza A has at least 18 different subtypes. These subtypes are named H1 through H18. H16 was discovered in 2004 on influenza A vir ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Influenza
Influenza, commonly known as "the flu", is an infectious disease caused by influenza viruses. Symptoms range from mild to severe and often include fever, runny nose, sore throat, muscle pain, headache, coughing, and fatigue. These symptoms begin from one to four days after exposure to the virus (typically two days) and last for about 2–8 days. Diarrhea and vomiting can occur, particularly in children. Influenza may progress to pneumonia, which can be caused by the virus or by a subsequent bacterial infection. Other complications of infection include acute respiratory distress syndrome, meningitis, encephalitis, and worsening of pre-existing health problems such as asthma and cardiovascular disease. There are four types of influenza virus, termed influenza viruses A, B, C, and D. Aquatic birds are the primary source of Influenza A virus (IAV), which is also widespread in various mammals, including humans and pigs. Influenza B virus (IBV) and Influenza C virus (ICV) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bacterial Neuraminidase
Bacterial neuraminidase is type of neuraminidase and a virulence factor for many bacteria including '' Bacteroides fragilis'' and '' Pseudomonas aeruginosa''. Its function is to cleave a sialic acid residue off ganglioside- GM1 (a modulator of cell surface and receptor activity) turning it into asialo-GM1 to which type 4 pili (attachment factors) bind preferentially. References {{Portal bar, Biology, border=no EC 3.2.1 Virulence factors ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Viral Neuraminidase
Viral neuraminidase is a type of neuraminidase found on the surface of influenza viruses that enables the virus to be released from the host cell. Neuraminidases are enzymes that cleave sialic acid (also called neuraminic acid) groups from glycoproteins. Neuraminidase inhibitors are antiviral agents that inhibit influenza viral neuraminidase activity and are of major importance in the control of influenza. Viral neuraminidases are the members of the glycoside hydrolase family 3CAZY GH_34which comprises enzymes with only one known activity; sialidase or neuraminidase . Neuraminidases cleave the terminal sialic acid residues from carbohydrate chains in glycoproteins. Sialic acid is a negatively charged sugar associated with the protein and lipid portions of lipoproteins. To infect a host cell, the influenza virus attaches to the exterior cell surface using hemagglutinin, a molecule found on the surface of the virus that binds to sialic acid groups. Sialic acids are found on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycoside Hydrolase Family 83
Hemagglutinin-neuraminidase refers to a single viral protein that has both hemagglutinin and (endo) neuraminidase activity. This is in contrast to the proteins found in influenza, where both functions exist but in two separate proteins. Its neuraminidase domain has the CAZy designation glycoside hydrolase family 83 (GH83). It does show a structural similarity to influenza viral neuraminidase and has a six-bladed beta-propeller structure. This Pfam entry also matches measles hemagglutinin (cd15467), which has a "dead" neuraminidase part repurposed as a receptor binding site. Hemagglutinin-neuraminidase allows the virus to stick to a potential host cell, and cut itself loose if necessary. Hemagglutinin-neuraminidase can be found in a variety of paramyxoviruses including mumps virus, human parainfluenza virus 3, and the avian pathogen Newcastle disease virus. Types include: * Mumps hemagglutinin-neuraminidase * Parainfluenza hemagglutinin-neuraminidase Parainfluenza hemaggl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycoside Hydrolase Family 58
In chemistry, a glycoside is a molecule in which a sugar is bound to another functional group via a glycosidic bond. Glycosides play numerous important roles in living organisms. Many plants store chemicals in the form of inactive glycosides. These can be activated by enzyme hydrolysis, which causes the sugar part to be broken off, making the chemical available for use. Many such plant glycosides are used as medications. Several species of ''Heliconius'' butterfly are capable of incorporating these plant compounds as a form of chemical defense against predators. In animals and humans, poisons are often bound to sugar molecules as part of their elimination from the body. In formal terms, a glycoside is any molecule in which a sugar group is bonded through its anomeric carbon to another group via a glycosidic bond. Glycosides can be linked by an O- (an ''O-glycoside''), N- (a ''glycosylamine''), S-(a ''thioglycoside''), or C- (a ''C-glycoside'') glycosidic bond. According to th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

_capsule.jpg)