|
Nanoribbon
Nanoribbon may refer to: * Graphene nanoribbons * Silicene nanoribbons * Boron nitride nanoribbons * Gallium(III) oxide nanoribbons * titanate nanoribbons - see titanium dioxide Titanium dioxide, also known as titanium(IV) oxide or titania , is the inorganic compound derived from titanium with the chemical formula . When used as a pigment, it is called titanium white, Pigment White 6 (PW6), or Colour Index Internationa ... * Phosphorene nanoribbons {{set index article ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Graphene Nanoribbons
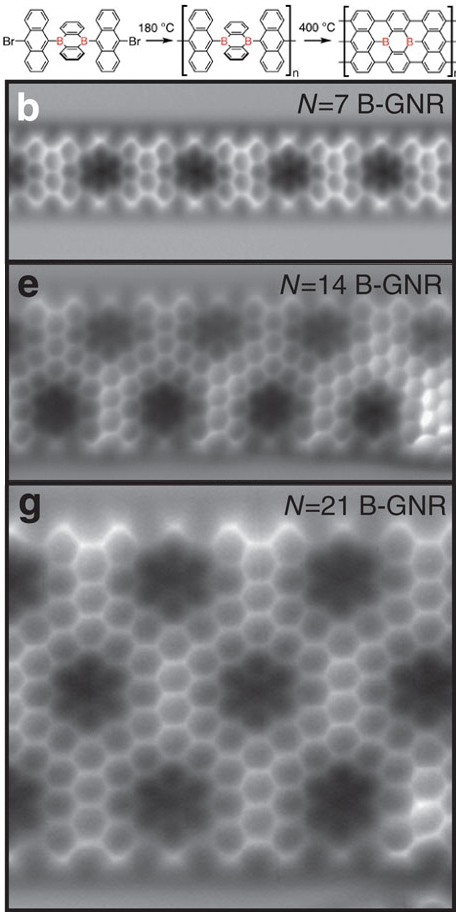
Graphene nanoribbons (GNRs, also called nano-graphene ribbons or nano-graphite ribbons) are strips of graphene with width less than 100 nm. Graphene ribbons were introduced as a theoretical model by Mitsutaka Fujita and coauthors to examine the edge and nanoscale size effect in graphene. Some earlier studies of graphitic ribbons within the area of conductive polymers in the field of synthetic metals include works by Kazuyoshi Tanaka, Tokio Yamabe and co-authors, Steven Kivelson and Douglas J. Klein. While Tanaka, Yamabe and Kivelson studied so-called zigzag and armchair edges of graphite, Klein introduced a different edge geometry that is frequently referred to as a bearded edge. Production Nanotomy Large quantities of width-controlled GNRs can be produced via graphite nanotomy, where applying a sharp diamond knife on graphite produces graphite nanoblocks, which can then be exfoliated to produce GNRs as shown by Vikas Berry. GNRs can also be produced by "unzipping" or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boron Nitride
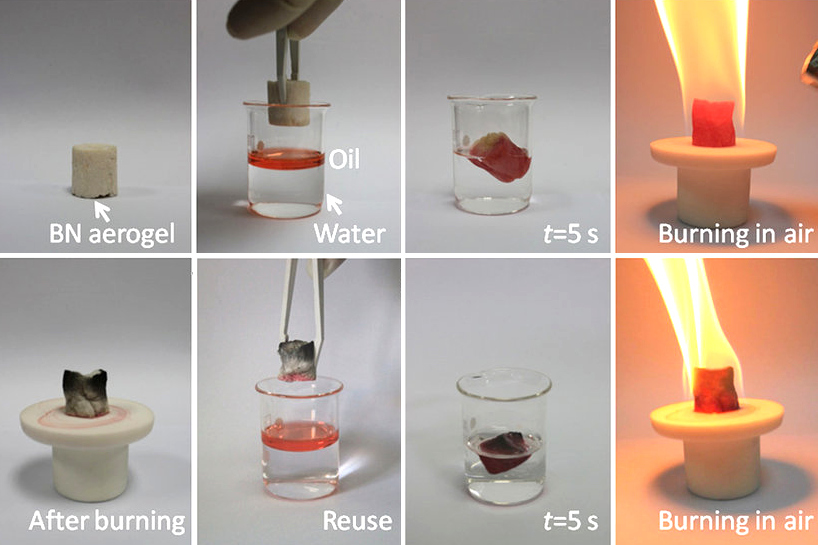
Boron nitride is a thermally and chemically resistant refractory compound of boron and nitrogen with the chemical formula B N. It exists in various crystalline forms that are isoelectronic to a similarly structured carbon lattice. The hexagonal form corresponding to graphite is the most stable and soft among BN polymorphs, and is therefore used as a lubricant and an additive to cosmetic products. The cubic ( zincblende aka sphalerite structure) variety analogous to diamond is called c-BN; it is softer than diamond, but its thermal and chemical stability is superior. The rare wurtzite BN modification is similar to lonsdaleite but slightly softer than the cubic form. Because of excellent thermal and chemical stability, boron nitride ceramics are used in high-temperature equipment and metal casting. Boron nitride has potential use in nanotechnology. History Boron nitride was discovered by chemistry teacher of the Liverpool Institute in 1842 via reduction of boric acid with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicene
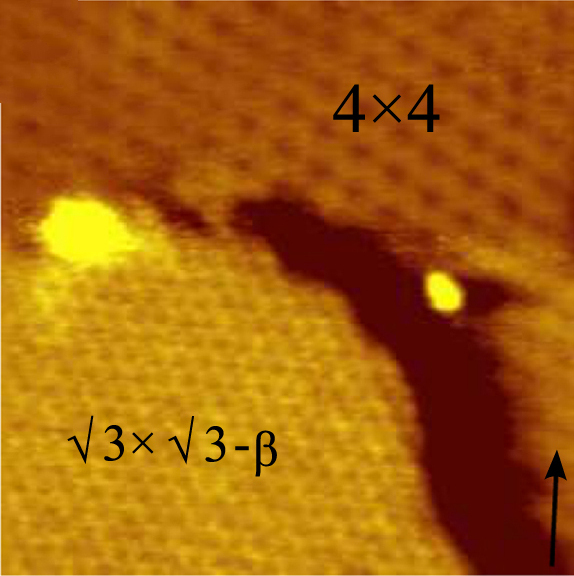
Silicene is a two-dimensional allotrope of silicon, with a hexagonal honeycomb structure similar to that of graphene. Contrary to graphene, silicene is not flat, but has a periodically buckled topology; the coupling between layers in silicene is much stronger than in multilayered graphene; and the oxidized form of silicene, 2D silica, has a very different chemical structure from graphene oxide. History Although theorists had speculated about the existence and possible properties of free-standing silicene, researchers first observed silicon structures that were suggestive of silicene in 2010. Using a scanning tunneling microscope they studied self-assembled silicene nanoribbons and silicene sheets deposited onto a silver crystal, Ag(110) and Ag(111), with atomic resolution. The images revealed hexagons in a honeycomb structure similar to that of graphene, which, however, were shown to originate from the silver surface mimicking the hexagons. Density functional theory (DFT) ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gallium(III) Oxide
Gallium(III) oxide is an inorganic compound and Wide-bandgap semiconductor, ultra-wide-bandgap semiconductor with the formula Gallium, Ga2trioxide, O3. It is actively studied for applications in power electronics, phosphors, and Gas detector, gas sensing. The compound has several polymorphism (materials science), polymorphs, of which the Monoclinic crystal system, monoclinic β-phase is the most stable. The β-phase’s bandgap of 4.7–4.9 eV and large-area, native substrates make it a promising competitor to Gallium nitride, GaN and Silicon carbide, SiC-based power electronics applications and Solar-blind technology, solar-blind UV Photodetector, photodetectors. The orthorhombic ĸ-Gallium, Ga2trioxide, O3 is the second most stable polymorph. The ĸ-phase has shown instability of subsurface doping density under thermal exposure. Ga2O3 exhibits reduced thermal conductivity and electron mobility by an order of magnitude compared to Gallium nitride, GaN and Silicon carbide, SiC, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Titanate
In chemistry, titanate usually refers to inorganic compounds composed of titanium oxides, or oxides containing the titanium element. Together with niobate, titanate salts form the Perovskite group. In some cases, the term is used more generally for any titanium-containing anion, e.g. iCl6sup>2− and i(CO)6sup>2−. This article focuses on the oxides. Many kinds of titanium oxides are known, and some are commercially important. Typically these materials are white, diamagnetic, high-melting, and insoluble in water. They are often prepared at high temperatures, e.g. using tube furnaces, from titanium dioxide. In virtually all cases, titanium achieves octahedral coordination geometry.. Orthotitanates Orthotitanates have the formula M2TiO4, where M is divalent. An example of such a material is magnesium titanate (Mg2TiO4), which adopts the spinel structure. Li2TiO3 is not considered an orthotitanate since it adopts the rock-salt structure and does not feature an identifiable t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Titanium Dioxide
Titanium dioxide, also known as titanium(IV) oxide or titania , is the inorganic compound derived from titanium with the chemical formula . When used as a pigment, it is called titanium white, Pigment White 6 (PW6), or Colour Index International, CI 77891. It is a white solid that is insoluble in water, although mineral forms can appear black. As a pigment, it has a wide range of applications, including paint, sunscreen, and food coloring. When used as a food coloring, it has E number E171. World production in 2014 exceeded 9 million tonnes. It has been estimated that titanium dioxide is used in two-thirds of all pigments, and pigments based on the oxide have been valued at a price of $13.2 billion. Structure In all three of its main dioxides, titanium exhibits Octahedral molecular geometry, octahedral geometry, being bonded to six oxide anions. The oxides in turn are bonded to three Ti centers. The overall crystal structures of rutile and anatase are tetragonal in symmetry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |