|
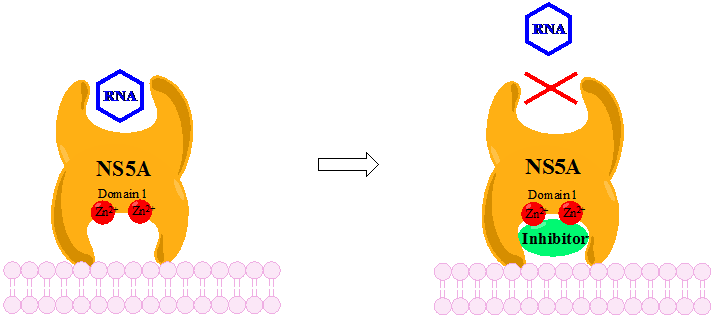
NS5A
Nonstructural protein 5A (NS5A) is a zinc-binding and proline-rich hydrophilic phosphoprotein that plays a key role in Hepatitis C virus RNA replication. It appears to be a dimeric form without ''trans''-membrane helices. Structure NS5A is derived from a large polyprotein that is translated from the HCV genome, and undergoes post-translation processing by nonstructural protein 3 (NS3) viral protease. Despite no inherent enzymatic activity being attributed to NS5A, its function is mediated through interaction with other nonstructural (NS) viral and cellular proteins. NS5A has two phosphorylated forms: p56 and p58, which differ in the electrophoretic mobility. p56 is basally phosphorylated by host cellular protein kinase at the center and near the C terminus, whereas p58 is a form of hyper-phosphorylated NS5A at the center of the serine-rich region. Protein mass spectrometry identified several phosphorylated serine residues in this region including serine 225, 229, 232, and 235 res ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Discovery And Development Of NS5A Inhibitors
Nonstructural protein 5A (NS5A) inhibitors are direct acting antiviral agents (DAAs) that target viral proteins, and their development was a culmination of increased understanding of the viral life cycle combined with advances in drug discovery technology. However, their mechanism of action is complex and not fully understood. NS5A inhibitors were the focus of much attention when they emerged as a part of the first curative treatment for hepatitis C virus (HCV) infections in 2014. Favorable characteristics have been introduced through varied structural changes, and structural similarities between NS5A inhibitors that are clinically approved are readily apparent. Despite the recent introduction of numerous new antiviral drugs, resistance is still a concern and these inhibitors are therefore always used in combination with other drugs. Hepatitis C virus HCV is a positive-sense single-stranded RNA virus that has been demonstrated to replicate in the hepatocytes of both humans and chim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Daclatasvir
Daclatasvir, sold under the brand name Daklinza, is an antiviral medication used in combination with other medications to treat hepatitis C (HCV). The other medications used in combination include sofosbuvir, ribavirin, and interferon, vary depending on the virus type and whether the person has cirrhosis. It is taken by mouth. Common side effects when used with sofusbivir and daclatasvir include headache, feeling tired, and nausea. With daclatasvir, sofosbuvir, and ribavirin the most common side effects are headache, feeling tired, nausea, and red blood cell breakdown. It should not be used with St. John's wort, rifampin, or carbamazepine. It works by inhibiting the HCV protein NS5A. Daclatasvir was approved for use in the European Union in 2014, and the United States and India in 2015. It is on the World Health Organization's List of Essential Medicines. The brand Daklinza is being withdrawn by Bristol Myers Squibb in countries where the drug is not typically prescribed, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Odalasvir
Odalasvir (INN, previously known as ACH-3102) is an investigational new drug in development for the treatment of hepatitis C. It is an NS5A inhibitor. The NS5A protein serves multiple functions at various stages of the viral life cycle, including viral replication. NS5A also plays a role in the development of interferon-resistance, a common cause of treatment failure. It is under development by Achillion Pharmaceuticals. See also * Discovery and development of NS5A inhibitors Nonstructural protein 5A (NS5A) inhibitors are direct acting antiviral agents (DAAs) that target viral proteins, and their development was a culmination of increased understanding of the viral life cycle combined with advances in drug discovery te ... References Benzimidazoles Carbamates NS5A inhibitors Experimental drugs Cyclophanes {{antiinfective-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Elbasvir
Elbasvir is a drug approved by the FDA in January 2016 for the treatment of hepatitis C. It was developed by Merck and completed Phase III trials, used in combination with the NS3/ 4A protease inhibitor grazoprevir under the trade name '' Zepatier'', either with or without ribavirin. Elbasvir is a highly potent and selective NS5A inhibitor of the hepatitis C virus NS5A replication complex. It has only been investigated as a combination product with other complementary hepatitis C antiviral drugs such as grazoprevir and MK-3682, and it is unclear whether elbasvir would show robust antiviral activity if it was administered by itself. Nevertheless, combination products of this type represent the most successful approach yet developed for actually curing hepatitis C, rather than merely slowing the progression of the disease. Side effects Side effects have only been assessed in the combination with grazoprevir; see Elbasvir/grazoprevir#Side effects. Interactions Elbasvir is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Velpatasvir
Velpatasvir is an NS5A inhibitor (by Gilead) which is used together with sofosbuvir in the treatment of hepatitis C infection of all six major genotypes. Side effects Side effects in studies occurred with similar frequencies as in people treated with placebo. Interactions Velpatasvir is both an inhibitor and a substrate of the transporter proteins P-glycoprotein (Pgp), ABCG2, OATP1B1 and OATP1B3. It is partly degraded by the liver enzymes CYP2B6, CYP2C8 and CYP3A4. Substances that are transported or inactivated by these proteins, or interfere with them, can interact with velpatasvir. In studies, this has been found for the HIV combination efavirenz/emtricitabine/tenofovir, which reduces the area under the curve (AUC) of velpatasvir by about 50%, and the CYP3A4 and Pgp inducer rifampicin, which reduces its AUC by about 80%, rendering it likely ineffective. Digoxin is eliminated by Pgp; its AUC is increased by about 30% in combination with velpatasvir and sofosbuvir (although ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Samatasvir
Samatasvir (IDX-719) is an experimental drug for the treatment of hepatitis C. It was originally developed by Idenix, and development has been continued by Merck & Co. following their acquisition of Idenix. Samatasvir has shown good results in Phase II trials. Samatasvir is a highly potent and selective inhibitor of the hepatitis C virus NS5A replication complex. While it showed promising results when administered as monotherapy, it is probable that samatasvir would be marketed as a combination product with other anti-hepatitis drugs to increase efficacy and reduce the chance of resistance developing, as with most other novel treatments for hepatitis C currently under development. Trials of samatasvir in combination with other antiviral drugs such as simeprevir are also underway. See also * Discovery and development of NS5A inhibitors Nonstructural protein 5A (NS5A) inhibitors are direct acting antiviral agents (DAAs) that target viral proteins, and their development was a c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ledipasvir
Ledipasvir is a drug for the treatment of hepatitis C that was developed by Gilead Sciences. After completing Phase III clinical trials, on February 10, 2014, Gilead filed for U.S. approval of a ledipasvir/sofosbuvir fixed-dose combination tablet for genotype 1 hepatitis C. The ledipasvir/sofosbuvir combination is a direct-acting antiviral agent that interferes with HCV replication and can be used to treat patients with genotypes 1a or 1b without PEG-interferon or ribavirin. Ledipasvir is an inhibitor of NS5A, a hepatitis C virus protein. Data presented at the 20th Conference on Retroviruses and Opportunistic Infections in March 2013 showed that a triple regimen of the nucleotide analog inhibitor sofosbuvir, ledipasvir, and ribavirin produced a 12-week post-treatment sustained virological response (SVR12) rate of 100% for both treatment-naive patients and prior non-responders with HCV genotype 1. The sofosbuvir/ledipasvir coformulation is being tested with and without riba ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ombitasvir
Ombitasvir is an antiviral drug for the treatment of hepatitis C virus (HCV) infection by AbbVie. In the United States, it is approved by the Food and Drug Administration for use in combination with paritaprevir, ritonavir and dasabuvir in the product Viekira Pak for the treatment of HCV genotype 1, and with paritaprevir and ritonavir in the product Technivie for the treatment of HCV genotype 4. Ombitasvir is an NS5A inhibitor that acts by inhibiting the HCV protein NS5A. See also * Discovery and development of NS5A inhibitors Nonstructural protein 5A (NS5A) inhibitors are direct acting antiviral agents (DAAs) that target viral proteins, and their development was a culmination of increased understanding of the viral life cycle combined with advances in drug discovery te ... References Further reading * * Breakthrough therapy Carbamates NS5A inhibitors Pyrrolidines {{Antiinfective-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
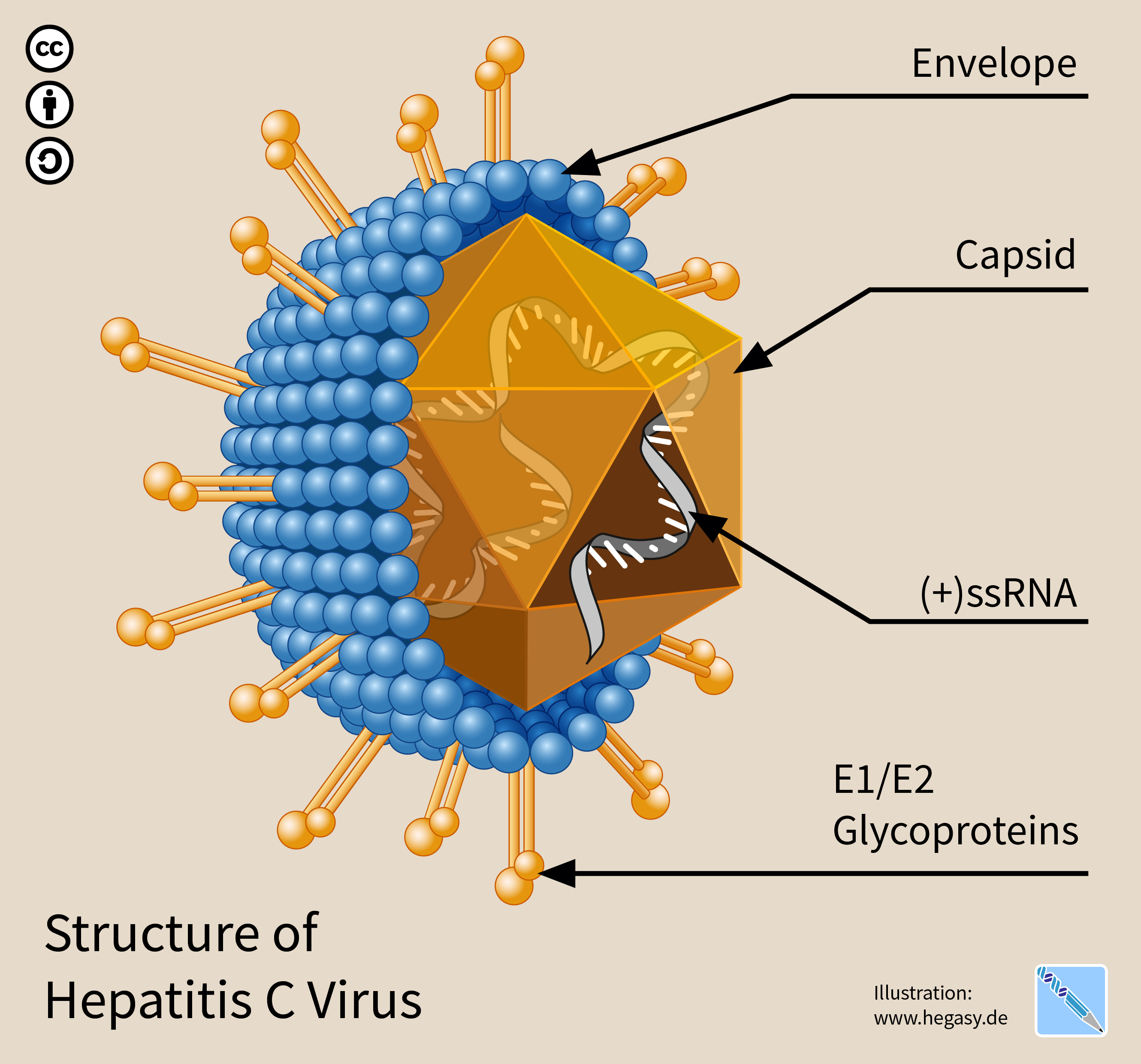
Hepatitis C Virus
The hepatitis C virus (HCV) is a small (55–65 nm in size), enveloped, positive-sense single-stranded RNA virus of the family '' Flaviviridae''. The hepatitis C virus is the cause of hepatitis C and some cancers such as liver cancer (hepatocellular carcinoma, abbreviated HCC) and lymphomas in humans. Taxonomy The hepatitis C virus belongs to the genus '' Hepacivirus'', a member of the family '' Flaviviridae''. Before 2011, it was considered to be the only member of this genus. However a member of this genus has been discovered in dogs: canine hepacivirus. There is also at least one virus in this genus that infects horses. Several additional viruses in the genus have been described in bats and rodents. Structure The hepatitis C virus particle consists of a lipid membrane envelope that is 55 to 65 nm in diameter. Two viral envelope glycoproteins, E1 and E2, are embedded in the lipid envelope. They take part in viral attachment and entry into the cell. Within the e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ravidasvir
Ravidasvir (PPI-668) is an investigational NS5A inhibitor (by Pharco Pharmaceuticals) in clinical trials for chronic hepatitis C genotype 4. Preliminary clinical trial results were announced in Nov 2015. In April 2017, press reports stated that a combination treatment involving ravidasvir and sofosbuvir had achieved a 97% clearup rate against hepatitis C in a clinical trial conducted in Malaysia and Thailand, and 100% in another conducted in Egypt. It has been granted conditional registration by the National Pharmaceutical Regulatory Agency (NPRA) of Malaysia. See also *Discovery and development of NS5A inhibitors Nonstructural protein 5A (NS5A) inhibitors are direct acting antiviral agents (DAAs) that target viral proteins, and their development was a culmination of increased understanding of the viral life cycle combined with advances in drug discovery te ... References Experimental drugs Gilead Sciences NS5A inhibitors {{antiinfective-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pibrentasvir
Pibrentasvir is an NS5A inhibitor antiviral agent. In the United States and Europe, it is approved for use with glecaprevir as the combination drug glecaprevir/pibrentasvir (trade name ''Mavyret'' in the US and ''Maviret'' in the EU) for the treatment of hepatitis C. It is sold by Abbvie AbbVie is an American publicly traded biopharmaceutical company founded in 2013. It originated as a spin-off of Abbott Laboratories. History On October 19, 2011, Abbott Laboratories announced its plan to separate into two publicly traded compani .... References {{Antiinfective-drug-stub NS5A inhibitors Fluoroarenes Benzimidazoles AbbVie brands Carbamates ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hepatitis C
Hepatitis C is an infectious disease caused by the hepatitis C virus (HCV) that primarily affects the liver; it is a type of viral hepatitis. During the initial infection people often have mild or no symptoms. Occasionally a fever, dark urine, abdominal pain, and yellow tinged skin occurs. The virus persists in the liver in about 75% to 85% of those initially infected. Early on, chronic infection typically has no symptoms. Over many years however, it often leads to liver disease and occasionally cirrhosis. In some cases, those with cirrhosis will develop serious complications such as liver failure, liver cancer, or dilated blood vessels in the esophagus and stomach. HCV is spread primarily by blood-to-blood contact associated with injection drug use, poorly sterilized medical equipment, needlestick injuries in healthcare, and transfusions. Using blood screening, the risk from a transfusion is less than one per two million. It may also be spread from an infected mother to h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |