|
NLRP7
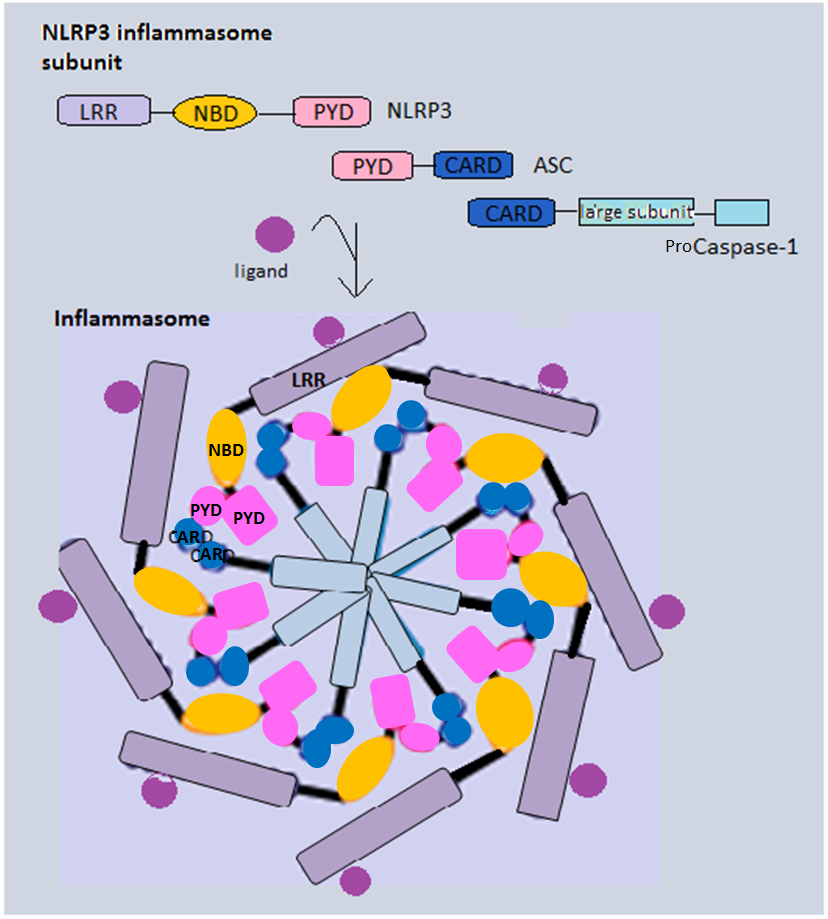
NACHT, LRR and PYD domains-containing protein 7 is a protein that in humans is encoded by the ''NLRP7'' gene. Function NALPs are cytoplasmic proteins that form a subfamily within the larger CATERPILLER protein family. Most short NALPs, such as NALP7, have an N-terminal pyrin (MEFV) domain (PYD), followed by a NACHT domain, a NACHT-associated domain (NAD), and a C-terminal leucine-rich repeat ( LRR) region. NALPs are implicated in the activation of proinflammatory caspases (e.g., CASP1) via their involvement in multiprotein complexes called inflammasome Inflammasomes are cytosolic Multiprotein complex, multiprotein oligomers of the innate immune system responsible for the activation of inflammatory responses. Activation and assembly of the inflammasome promotes proteolytic cleavage, maturation an ...s. References Further reading * * * * * * * * * * * LRR proteins NOD-like receptors {{gene-19-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NACHT Domain
The NACHT domain is an evolutionarily conserved protein domain. This NTPase domain is found in apoptosis proteins as well as those involved in MHC transcription. Its name reflects some of the proteins that contain it: NAIP (NLP family apoptosis inhibitor protein), CIITA (that is, C2TA or MHC class II transcription activator), HET-E (incompatibility locus protein from ''Podospora anserina'') and TEP1 (that is, TP1 or telomerase-associated protein). The NACHT domain contains 300 to 400 amino acids. It is a predicted nucleoside-triphosphatase (NTPase) domain, which is found in animal, fungal and bacterial proteins. It is found in association with other domains, such as the CARD domain (), the pyrin domain (), the HEAT repeat domain (), the WD40 repeat (), the leucine-rich repeat (LRR) or the BIR repeat (). The NACHT domain consists of seven distinct conserved motifs, including the ATP/GTPase specific P-loop, the Mg2+-binding site ( Walker A and B motifs, respective ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid resid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gene
In biology, the word gene (from , ; "... Wilhelm Johannsen coined the word gene to describe the Mendelian units of heredity..." meaning ''generation'' or ''birth'' or ''gender'') can have several different meanings. The Mendelian gene is a basic unit of heredity and the molecular gene is a sequence of nucleotides in DNA that is transcribed to produce a functional RNA. There are two types of molecular genes: protein-coding genes and noncoding genes. During gene expression, the DNA is first copied into RNA. The RNA can be directly functional or be the intermediate template for a protein that performs a function. The transmission of genes to an organism's offspring is the basis of the inheritance of phenotypic traits. These genes make up different DNA sequences called genotypes. Genotypes along with environmental and developmental factors determine what the phenotypes will be. Most biological traits are under the influence of polygenes (many different genes) as well as g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-terminus
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amine group is bonded to the carboxylic group of another amino acid, making it a chain. That leaves a free carboxylic group at one end of the peptide, called the C-terminus, and a free amine group on the other end called the N-terminus. By convention, peptide sequences are written N-terminus to C-terminus, left to right (in LTR writing systems). This correlates the translation direction to the text direction, because when a protein is translated from messenger RNA, it is created from the N-terminus to the C-terminus, as amino acids are added to the carboxyl end of the protein. Chemistry Each amino acid has an amine group and a carboxylic group. Amino acids link to one another by peptide bonds which form through a dehydration reaction ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MEFV
''MEFV'' (Mediterranean fever) is a human gene that provides instructions for making a protein called pyrin (also known as marenostrin). Pyrin is produced in certain white blood cells (neutrophils, eosinophils and monocytes) that play a role in inflammation and in fighting infection. Inside these white blood cells, pyrin is found with the cytoskeleton, the structural framework that helps to define the shape, size, and movement of a cell. Pyrin's protein structure also allows it to interact with other molecules involved in fighting infection and in the inflammatory response. Although pyrin's function is not fully understood, it likely assists in keeping the inflammation process under control. Research indicates that pyrin helps regulate inflammation by interacting with the cytoskeleton. Pyrin may direct the migration of white blood cells to sites of inflammation and stop or slow the inflammatory response when it is no longer needed. The ''MEFV'' gene is located on the short (p) arm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-terminus
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is translated from messenger RNA, it is created from N-terminus to C-terminus. The convention for writing peptide sequences is to put the C-terminal end on the right and write the sequence from N- to C-terminus. Chemistry Each amino acid has a carboxyl group and an amine group. Amino acids link to one another to form a chain by a dehydration reaction which joins the amine group of one amino acid to the carboxyl group of the next. Thus polypeptide chains have an end with an unbound carboxyl group, the C-terminus, and an end with an unbound amine group, the N-terminus. Proteins are naturally synthesized starting from the N-terminus and ending at the C-terminus. Function C-terminal retention signals While the N-terminus of a protein often co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leucine-rich Repeat
A leucine-rich repeat (LRR) is a protein structural motif that forms an α/β horseshoe fold. It is composed of repeating 20–30 amino acid stretches that are unusually rich in the hydrophobic amino acid leucine. These tandem repeats commonly fold together to form a solenoid protein domain, termed leucine-rich repeat domain. Typically, each repeat unit has beta strand-turn-alpha helix structure, and the assembled domain, composed of many such repeats, has a horseshoe shape with an interior parallel beta sheet and an exterior array of helices. One face of the beta sheet and one side of the helix array are exposed to solvent and are therefore dominated by hydrophilic residues. The region between the helices and sheets is the protein's hydrophobic core and is tightly sterically packed with leucine residues. Leucine-rich repeats are frequently involved in the formation of protein–protein interactions. Examples Leucine-rich repeat motifs have been identified in a large num ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caspase
Caspases (cysteine-aspartic proteases, cysteine aspartases or cysteine-dependent aspartate-directed proteases) are a family of protease enzymes playing essential roles in programmed cell death. They are named caspases due to their specific cysteine protease activity – a cysteine in its active site nucleophilically attacks and cleaves a target protein only after an aspartic acid residue. As of 2009, there are 12 confirmed caspases in humans and 10 in mice, carrying out a variety of cellular functions. The role of these enzymes in programmed cell death was first identified in 1993, with their functions in apoptosis well characterised. This is a form of programmed cell death, occurring widely during development, and throughout life to maintain cell homeostasis. Activation of caspases ensures that the cellular components are degraded in a controlled manner, carrying out cell death with minimal effect on surrounding tissues. Caspases have other identified roles in programmed ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CASP1
Caspase-1/Interleukin-1 converting enzyme (ICE) is an evolutionarily conserved enzyme that proteolytically cleaves other proteins, such as the precursors of the inflammatory cytokines interleukin 1β and interleukin 18 as well as the pyroptosis inducer Gasdermin D, into active mature peptides. It plays a central role in cell immunity as an inflammatory response initiator. Once activated through formation of an inflammasome complex, it initiates a proinflammatory response through the cleavage and thus activation of the two inflammatory cytokines, interleukin 1β (IL-1β) and interleukin 18 (IL-18) as well as pyroptosis, a programmed lytic cell death pathway, through cleavage of Gasdermin D. The two inflammatory cytokines activated by Caspase-1 are excreted from the cell to further induce the inflammatory response in neighboring cells. Cellular expression Caspase-1 is evolutionarily conserved in many eukaryotes of the Kingdom Animalia. Due to its role in the inflammatory immun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inflammasome
Inflammasomes are cytosolic Multiprotein complex, multiprotein oligomers of the innate immune system responsible for the activation of inflammatory responses. Activation and assembly of the inflammasome promotes proteolytic cleavage, maturation and secretion of pro-inflammatory cytokines Interleukin 1 beta, interleukin 1β (IL-1β) and Interleukin 18, interleukin 18 (IL-18), as well as cleavage of Gasdermin D, Gasdermin-D. The N-terminal fragment resulting from this cleavage induces a pro-inflammatory form of programmed cell death distinct from apoptosis, referred to as pyroptosis, and is responsible for secretion of the mature cytokines, presumably through the formation of pores in the plasma membrane. Inflammasome activation is initiated by different kinds of cytosolic pattern recognition receptors (PRRs) that respond to either microbe-derived pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs) generated by the host cell. Pattern recogn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
LRR Proteins
{{dab ...
LRR may refer to: * Laminated root rot, a root disease in conifers *Leucine-rich repeat, a type of protein domain * LoadingReadyRun, a Canadian comedy troupe * Long Range Radar * '' Long River Review'', a literary magazine of the University of Connecticut * Low rolling resistance tires, a type of tires designed for fuel efficiency * Light Reaction Regiment, the Philippine Army counter-terrorist unit modeled after the U.S. Army Delta Force and British SAS * Loose Round Robin, Warp Scheduling See also * LR (other) * Lrrr (other) LRRR may refer to: * Laser Ranging Retroreflector, for Lunar laser ranging * Lrrr, a character in the TV series ''Futurama'' See also * LRR (other) {{disambiguation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |