|
N-Hydroxysuccinimide
''N''-Hydroxysuccinimide (NHS) is an organic compound with the formula (CH2CO)2NOH. It is a white solid that is used as a reagent for preparing active esters in peptide synthesis. It can be synthesized by heating succinic anhydride with hydroxylamine or hydroxylamine hydrochloride. Activating reagent NHS is commonly found in organic chemistry or biochemistry where it is used as an activating reagent for carboxylic acids. Activated acids (carboxylates) can react with amines to form amides for example, whereas a normal carboxylic acid would just form a salt with an amine. Use A common way to synthesize an NHS-activated acid is to mix NHS with the desired carboxylic acid and a small amount of an organic base in an anhydrous solvent. A coupling reagent such as dicyclohexylcarbodiimide (DCC) or 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) is then added to form a highly reactive activated acid intermediate. NHS reacts to create a less labile activated acid. The group is us ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
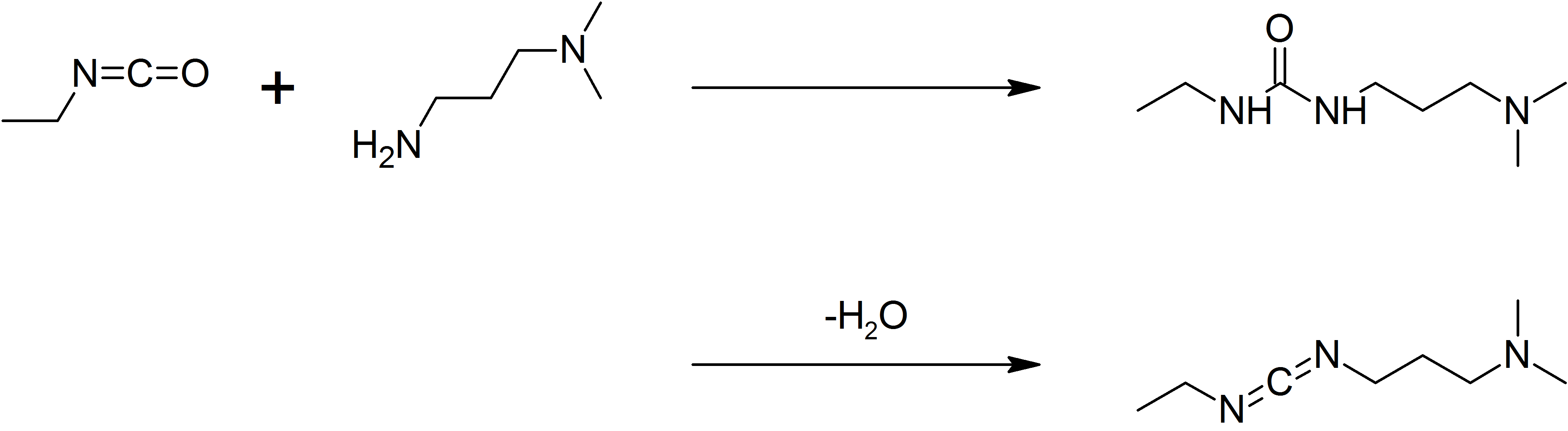
1-ethyl-3-(3-dimethylaminopropyl)carbodiimide
1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC, EDAC or EDCI) is a water-soluble carbodiimide usually handled as the hydrochloride. It is typically employed in the 4.0-6.0 pH range. It is generally used as a carboxyl activating agent for the coupling of primary amines to yield amide bonds. While other carbodiimides like dicyclohexylcarbodiimide (DCC) or diisopropylcarbodiimide (DIC) are also employed for this purpose, EDC has the advantage that the urea byproduct formed (often challenging to remove in the case of DCC or DIC) can be washed away from the amide product using dilute acid. Additionally, EDC can also be used to activate phosphate groups in order to form phosphomonoesters and phosphodiesters. Common uses for this carbodiimide include peptide synthesis, protein crosslinking to nucleic acids, but also in the preparation of immunoconjugates. EDC is often used in combination with ''N''-hydroxysuccinimide (NHS) for the immobilisation of large biomolecules. Recent w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Succinimide
Succinimide is an organic compound with the formula (CH2)2(CO)2NH. This white solid is used in a variety of organic syntheses, as well as in some industrial silver plating processes. The compound is classified as a cyclic imide. It may be prepared by thermal decomposition of ammonium succinate. Succinimides Succinimides refers to compounds that contain the succinimide group. These compounds have some notable uses. Several succinimides are used as anticonvulsant drugs, including ethosuximide, phensuximide, and methsuximide. Succinimides are also used to form covalent bonds between proteins or peptides and plastics, which is useful in a variety of assay techniques. See also * Succinic anhydride * N-Hydroxysuccinimide, ''N''-Hydroxysuccinimide * N-Bromosuccinimide, ''N''-Bromosuccinimide References {{Authority control Succinimides, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CRC Handbook Of Chemistry And Physics
The ''CRC Handbook of Chemistry and Physics'' is a comprehensive one-volume reference resource for science research. First published in 1914, it is currently () in its 105th edition, published in 2024. It is known colloquially among chemists as the "Rubber Bible", as CRC originally stood for "Chemical Rubber Company". As late as the 1962–1963 edition (3604 pages), the ''Handbook'' contained myriad information for every branch of science and engineering. Sections in that edition include: Mathematics, Properties and Physical Constants, Chemical Tables, Properties of Matter, Heat, Hygrometric and Barometric Tables, Sound, Quantities and Units, and Miscellaneous. ''Mathematical Tables from Handbook of Chemistry and Physics'' was originally published as a supplement to the handbook up to the 9th edition (1952); afterwards, the 10th edition (1956) was published separately as '' CRC Standard Mathematical Tables''. Earlier editions included sections such as "Antidotes of Poisons", ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anhydrous
A substance is anhydrous if it contains no water. Many processes in chemistry can be impeded by the presence of water; therefore, it is important that water-free reagents and techniques are used. In practice, however, it is very difficult to achieve perfect dryness; anhydrous compounds gradually absorb water from the atmosphere so they must be stored carefully. Solids Many salts and solids can be dried using heat, or under vacuum. Desiccators can also be used to store reagents in dry conditions. Common desiccants include phosphorus pentoxide and silica gel. Chemists may also require dry glassware for sensitive reactions. This can be achieved by drying glassware in an oven, by flame, or under vacuum. Dry solids can be produced by freeze-drying, which is also known as lyophilization. Liquids or solvents In many cases, the presence of water can prevent a reaction from happening, or cause undesirable products to form. To prevent this, anhydrous solvents must be used when perform ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1-hydroxy-7-azabenzotriazole
1-Hydroxy-7-azabenzotriazole (HOAt) is a triazole used as a peptide coupling reagent. It suppresses racemization In chemistry, racemization is a conversion, by heat or by chemical reaction, of an optically active compound into a racemic (optically inactive) form. This creates a 1:1 molar ratio of enantiomers and is referred to as a racemic mixture (i.e. cont ... that can otherwise occur during the reaction. HOAt has a melting point of 213-216 °C. References Peptide coupling reagents Triazolopyridines Reagents for biochemistry Hydroxylamines {{heterocyclic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxybenzotriazole
Hydroxybenzotriazole (abbreviated HOBt) is an organic compound with the formula . It is a derivative of benzotriazole. It is a white crystalline powder, which as a commercial product contains some water (~11.7% wt as the HOBt monohydrate crystal). Anhydrous HOBt is explosive. It is mainly used to suppress the racemization and to improve the efficiency of peptide synthesis. Use in peptide synthesis Automated peptide synthesis involves the condensation of the amino group of protected amino acids with the activated ester. HOBt is used to produce such activated esters which react with amines at ambient temperature to give amides. HOBt is also used for the synthesis of amides from carboxylic acids aside from amino acids. These substrates may not be convertible to the acyl chlorides. Safety Due to reclassification as UN0508, a class 1.3C explosive, hydroxybenzotriazole and its monohydrate crystal are no longer allowed to be transported by sea or air as per 49CFR (USDOT hazard ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biosensor
A biosensor is an analytical device, used for the detection of a chemical substance, that combines a biological component with a physicochemical detector. The ''sensitive biological element'', e.g. tissue, microorganisms, organelles, cell receptors, enzymes, antibodies, nucleic acids, etc., is a biologically derived material or biomimetic component that interacts with, binds with, or recognizes the analyte under study. The biologically sensitive elements can also be created by biological engineering. The ''transducer'' or the ''detector element'', which transforms one signal into another one, works in a physicochemical way: optical, piezoelectric, electrochemical, electrochemiluminescence etc., resulting from the interaction of the analyte with the biological element, to easily measure and quantify. The biosensor reader device connects with the associated electronics or signal processors that are primarily responsible for the display of the results in a user-friendly way. Thi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different molecules known as product (chemistry), products. Almost all metabolism, metabolic processes in the cell (biology), cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme, pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts include Ribozyme, catalytic RNA molecules, also called ribozymes. They are sometimes descr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorescein
Fluorescein is an organic compound and dye based on the xanthene tricyclic structural motif, formally belonging to Triarylmethane dye, triarylmethine dyes family. It is available as a dark orange/red powder slightly soluble in water and alcohol. It is used as a fluorescent Flow tracer, tracer in many applications. The color of its aqueous solutions is green by reflection and orange by transmission (its spectral properties are dependent on pH of the solution), as can be noticed in spirit level, bubble levels, for example, in which fluorescein is added as a colorant to the Alcohol (chemistry), alcohol filling the tube in order to increase the visibility of the air bubble contained within. More concentrated solutions of fluorescein can even appear red (because under these conditions nearly all incident emission is re-absorbed by the solution). It is on the WHO Model List of Essential Medicines, World Health Organization's List of Essential Medicines. Uses Fluorescein sodium, t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metabolic reactions, DNA replication, Cell signaling, responding to stimuli, providing Cytoskeleton, structure to cells and Fibrous protein, organisms, and Intracellular transport, transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the Nucleic acid sequence, nucleotide sequence of their genes, and which usually results in protein folding into a specific Protein structure, 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called pep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dicyclohexylcarbodiimide
is an organic compound with the chemical formula (C6H11N)2C. It is a waxy white solid with a sweet odor. Its primary use is to couple amino acids during artificial peptide synthesis. The low melting point of this material allows it to be melted for easy handling. It is highly soluble in dichloromethane, tetrahydrofuran, acetonitrile and dimethylformamide, but insoluble in water. Structure and spectroscopy The C−N=C=N−C core of carbodiimides (N=C=N) is linear, being related to the structure of allene. The molecule has idealized C2 symmetry. The N=C=N moiety gives characteristic IR spectroscopic signature at 2117 cm−1. The 15N NMR spectrum shows a characteristic shift of 275 ppm upfield of nitric acid and the 13C NMR spectrum features a peak at about 139 ppm downfield from TMS. Preparation DCC is produced by the decarboxylation of cyclohexylisocyanate using phosphine oxides as a catalyst: :2 C6H11NCO → (C6H11N)2C + CO2 Alternative catalysts for th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |