|
N-Hydroxypiperidine
''N''-Hydroxypiperidine (also known as 1-piperidinol and 1-hydroxypiperidine) is the chemical compound with formula C5H11NO. It is a hydroxylated derivative of the heterocyclic compound piperidine. Preparation ''N''-Hydroxypiperidine can be prepared from the application of ''meta''-chloroperoxybenzoic acid and methanol to the tertiary amine product of acrylonitrile and piperidine Piperidine is an organic compound with the molecular formula (CH2)5NH. This heterocyclic amine consists of a six-membered ring containing five methylene bridges (–CH2–) and one amine bridge (–NH–). It is a colorless liquid with an odor de ..., followed by heating with acetone of the resulting tertiary ''N''-oxide. Reactions ''N''-Hydroxypiperidine is a secondary amine, which can undergo an oxidation reaction with hydrogen peroxide in methanol as the solvent. This produces a nitrone, which is heteroatomic equivalent to a ketone with a nitrogen instead of an alpha carbon. Competing elimination ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Piperidine
Piperidine is an organic compound with the molecular formula (CH2)5NH. This heterocyclic amine consists of a six-membered ring containing five methylene bridges (–CH2–) and one amine bridge (–NH–). It is a colorless liquid with an odor described as objectionable, typical of amines. The name comes from the genus name '' Piper'', which is the Latin word for pepper. Although piperidine is a common organic compound, it is best known as a representative structure element within many pharmaceuticals and alkaloids, such as natural-occurring solenopsins. Production Piperidine was first reported in 1850 by the Scottish chemist Thomas Anderson and again, independently, in 1852 by the French chemist Auguste Cahours, who named it. Both of them obtained piperidine by reacting piperine with nitric acid. Industrially, piperidine is produced by the hydrogenation of pyridine, usually over a molybdenum disulfide catalyst: : C5H5N + 3 H2 → C5H10NH Pyridine can also be reduce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
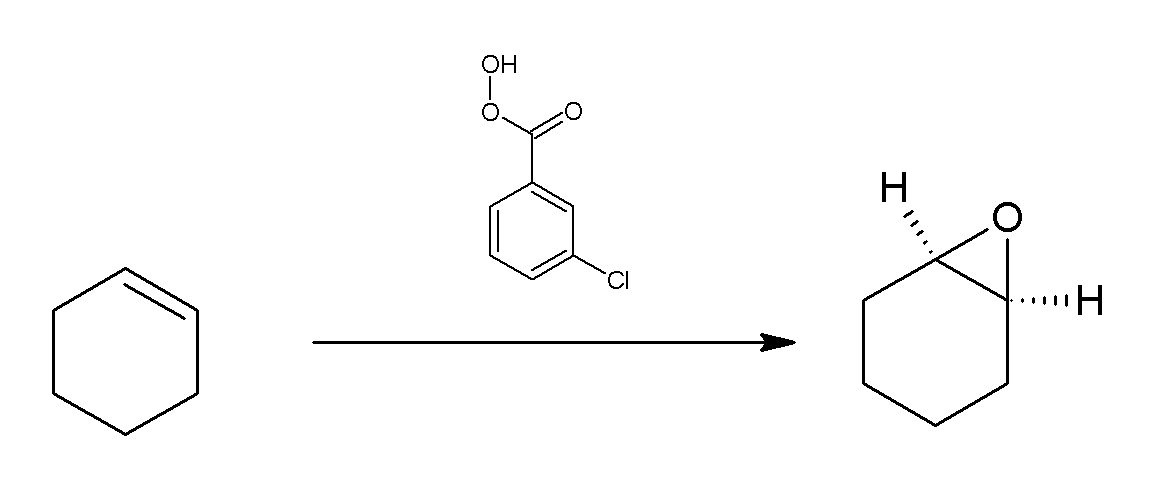
Meta-Chloroperoxybenzoic Acid
''meta''-Chloroperoxybenzoic acid (mCPBA or ''m''CPBA) is a peroxycarboxylic acid. It is a white solid often used widely as an oxidant in organic synthesis. mCPBA is often preferred to other peroxy acids because of its relative ease of handling. mCPBA is a strong oxidizing agent that may cause fire upon contact with flammable material. Preparation and purification mCPBA can be prepared by reacting m-chlorobenzoyl chloride with a basic solution of hydrogen peroxide, followed by acidification. It is sold commercially as a shelf-stable mixture that is less than 72% mCPBA, with the balance made up of ''m''-chlorobenzoic acid (10%) and water. The peroxyacid can be purified by washing the commercial material with a sodium hydroxide and potassium phosphate solution buffered at pH = 7.5. Peroxyacids are generally slightly less acidic than their carboxylic acid counterparts, so the acid impurity can be extracted if the pH is carefully controlled. The purified material is reasonably sta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical compound and the simplest aliphatic Alcohol (chemistry), alcohol, with the chemical formula (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a light, Volatility (chemistry), volatile, colorless and flammable liquid with a distinctive alcoholic odor similar to that of ethanol (potable alcohol), but is more acutely toxic than the latter. Methanol acquired the name wood alcohol because it was once produced through destructive distillation of wood. Today, methanol is mainly produced industrially by hydrogenation of carbon monoxide. Methanol consists of a methyl group linked to a polar hydroxyl group. With more than 20 million tons produced annually, it is used as a Precursor (chemistry), precursor to other commodity chemicals, including formaldehyde, acetic acid, methyl tert-butyl ether, methyl ''tert''-butyl ether, methyl benzoate, anisole, peroxyacids, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acrylonitrile
Acrylonitrile is an organic compound with the formula and the structure . It is a colorless, volatile liquid. It has a pungent odor of garlic or onions. Its molecular structure consists of a vinyl group () linked to a nitrile (). It is an important monomer for the manufacture of useful plastics such as polyacrylonitrile. It is reactive and toxic at low doses. Acrylonitrile is one of the components of ABS plastic (acrylonitrile butadiene styrene). Structure and basic properties Acrylonitrile is an organic compound with the formula and the structure . It is a colorless, volatile liquid although commercial samples can be yellow due to impurities. It has a pungent odor of garlic or onions. Its molecular structure consists of a vinyl group () linked to a nitrile (). It is an important monomer for the manufacture of useful plastics such as polyacrylonitrile. It is reactive and toxic at low doses. Production Acrylonitrile was first synthesized by the French chemist Charle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-oxide
In chemistry, an amine oxide, also known as an amine ''N''-oxide or simply ''N''-oxide, is a chemical compound that has the chemical formula . It contains a nitrogen-oxygen coordinate covalent bond with three additional hydrogen and/or substituent-groups attached to nitrogen. Sometimes it is written as or, alternatively, as . In the strict sense, the term ''amine oxide'' applies only to oxides of tertiary amines. Sometimes it is also used for the analogous derivatives of primary and secondary amines. Examples of amine oxides include pyridine-''N''-oxide, a water-soluble crystalline solid with melting point 62–67 °C, and ''N''-methylmorpholine ''N''-oxide, which is an oxidant. Applications Amine oxides are surfactants commonly used in consumer products such as shampoos, conditioners, detergents, and hard surface cleaners. Alkyl dimethyl amine oxide (chain lengths C10–C16) is the most commercially used amine oxide. They are considered a high production volume class o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |