|
Myelin-associated Glycoprotein
Myelin-associated glycoprotein (MAG), or Siglec-4 is a type 1 transmembrane protein, a glycoprotein localized in periaxonal Schwann cell and oligodendrocyte membranes, where it plays a role in glial-axonal interactions. MAG is a member of the SIGLEC family of proteins and is a functional ligand of the NOGO-66 receptor, NgR. MAG is believed to be involved in myelination during remyelination (nerve regeneration) in the peripheral nervous system (PNS) and is vital for the long-term survival of the myelinated axons following myelinogenesis. In the CNS MAG is one of three main myelin-associated inhibitors of axonal regeneration after injury, making it an important protein for future research on neurogenesis in the CNS. Structure MAG is a 100 kDA glycoprotein. Uncleaved MAG is a complete transmembrane form, which acts as a signaling and adhesion molecule. MAG can also act as a signaling molecule as a soluble protein after it has been proteolytically shed. This form of the prot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
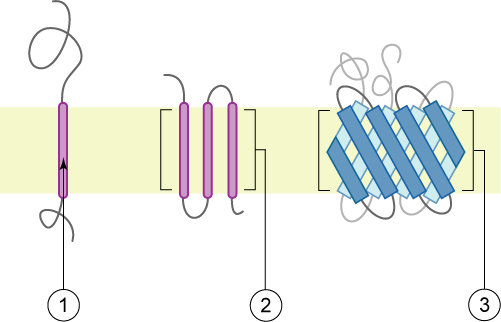
Type 1 Transmembrane Protein
A single-pass membrane protein also known as single-spanning protein or bitopic protein is a transmembrane protein that spans the lipid bilayer only once. These proteins may constitute up to 50% of all transmembrane proteins, depending on the organism, and contribute significantly to Interactome, the network of interactions between different proteins in cells, including interactions via transmembrane alpha helix, alpha helices. They usually include one or several water-soluble Protein domain, domains situated at the different sides of biological membranes, for example in single-pass transmembrane receptors. Some of them are small and serve as regulatory or structure-stabilizing subunits in large multi-protein transmembrane complexes, such as photosystems or the respiratory chain. More than 2300 single-pass membrane proteins were identified in the human genome. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Immunoglobulin Domain
The immunoglobulin domain, also known as the immunoglobulin fold, is a type of protein domain that consists of a 2-layer sandwich of 7-9 antiparallel β-strands arranged in two β-sheets with a Greek key topology, consisting of about 125 amino acids. The backbone switches repeatedly between the two β-sheets. Typically, the pattern is (N-terminal β-hairpin in sheet 1)-(β-hairpin in sheet 2)-(β-strand in sheet 1)-(C-terminal β-hairpin in sheet 2). The cross-overs between sheets form an "X", so that the N- and C-terminal hairpins are facing each other. Members of the immunoglobulin superfamily are found in hundreds of proteins of different functions. Examples include antibodies, the giant muscle kinase titin, and receptor tyrosine kinases. Immunoglobulin-like domains may be involved in protein–protein and protein–ligand interactions. Examples Human genes encoding proteins containing the immunoglobulin domain include: * A1BG * ACAM * ADAMTSL1 * ADAMTSL3 * AGER * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anti-MAG Peripheral Neuropathy
Anti-MAG peripheral neuropathy is a specific type of peripheral neuropathy in which the person's own immune system attacks cells that are specific in maintaining a healthy nervous system. As these cells are destroyed by antibody, antibodies, the neuron, nerve cells in the surrounding region begin to lose function and create many problems in both sensory and motor function. Specifically, antibodies against myelin-associated glycoprotein (MAG) damage Schwann cells. While the disorder occurs in only 10% of those afflicted with peripheral neuropathy, people afflicted have symptoms such as muscle weakness, sensory problems, and other motor deficits usually starting in the form of a tremor of the hands or trouble walking. There are, however, multiple Therapy, treatments that range from simple exercises in order to build strength to targeted drug treatments that have been shown to improve function in people with this type of peripheral neuropathy. Background Myelination by Schwann cells ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Myelin Oligodendrocyte Glycoprotein
Myelin oligodendrocyte glycoprotein (MOG) is a glycoprotein believed to be important in the myelination of nerves in the central nervous system (CNS). In humans this protein is encoded by the ''MOG'' gene. It is speculated to serve as a necessary "adhesion molecule" to provide structural integrity to the myelin sheath and is known to develop late on the oligodendrocyte. Molecular function While the primary molecular function of MOG is not yet known, its likely role with the myelin sheath is either in sheath "completion and/or maintenance". More specifically, MOG is speculated to be "necessary" as an "adhesion molecule" on the myelin sheath of the CNS to provide the structural integrity of the myelin sheath.Berger, T., Innsbruck Medical University Dept. of Neurology interviewed by S. Gillooly, Nov. 24, 2008." MOG's cDNA coding region in humans have been shown to be "highly homologous" to rats, mice, and bovine, and hence highly conserved. This suggests "an important biological ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Myelinogenesis
Myelination, or myelinogenesis, is the formation and development of myelin sheaths in the nervous system, typically initiated in late prenatal neurodevelopment and continuing throughout postnatal development. The term ''myelinogenesis'' is also sometimes used to differentiate the very early stages of embryonic myelination. Myelin is formed by oligodendrocytes in the central nervous system and Schwann cells in the peripheral nervous system. Myelination continues throughout the lifespan to support learning and memory via neural circuit plasticity as well as remyelination following injury. Successful myelination of axons increases action potential speed by enabling saltatory conduction, which is essential for timely signal conduction between spatially separate brain regions, as well as provides metabolic support to neurons. Stages Myelin is formed by oligodendrocytes in the central nervous system and Schwann cells in the peripheral nervous system. Therefore, the first stage of m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Myelin
Myelin Sheath ( ) is a lipid-rich material that in most vertebrates surrounds the axons of neurons to insulate them and increase the rate at which electrical impulses (called action potentials) pass along the axon. The myelinated axon can be likened to an electrical wire (the axon) with insulating material (myelin) around it. However, unlike the plastic covering on an electrical wire, myelin does not form a single long sheath over the entire length of the axon. Myelin ensheaths part of an axon known as an internodal segment, in multiple myelin layers of a tightly regulated internodal length. The ensheathed segments are separated at regular short unmyelinated intervals, called nodes of Ranvier. Each node of Ranvier is around one micrometre long. Nodes of Ranvier enable a much faster rate of conduction known as saltatory conduction where the action potential recharges at each node to jump over to the next node, and so on till it reaches the axon terminal. At the terminal the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rho-associated Protein Kinase
Rho-associated protein kinase or Rho-associated coiled-coil kinase (ROCK) is a kinase belonging to the AGC (PKA/ PKG/PKC) family of serine-threonine specific protein kinases. It is involved mainly in regulating the shape and movement of cells by acting on the cytoskeleton. ROCKs (ROCK1 and ROCK2) occur in mammals (human, rat, mouse, cow), zebrafish, ''Xenopus'', invertebrates (''C. elegans'', mosquito, ''Drosophila'') and chicken. Human ROCK1 has a molecular mass of 158 kDa and is a major downstream effector of the small GTPase RhoA. Mammalian ROCK consists of a kinase domain, a coiled-coil region and a Pleckstrin homology (PH) domain, which reduces the kinase activity of ROCKs by an autoinhibitory intramolecular fold if RhoA-GTP is not present. Rat ROCKs were discovered as the first effectors of Rho and they induce the formation of stress fibers and focal adhesions by phosphorylating MLC (myosin light chain). Due to this phosphorylation, the actin binding of myosin II and, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reticulon 4
Reticulon 4, also known as Neurite outgrowth inhibitor or Nogo, is a protein that in humans is encoded by the ''RTN4'' gene that has been identified as an inhibitor of neurite outgrowth specific to the central nervous system. During neural development Nogo is expressed mainly by neurons and provides an inhibitory signal for the migration and sprouting of CNS endothelial (tip) cells, thereby restricting blood vessel density. This gene belongs to the family of reticulon-encoding genes. Reticulons are associated with the endoplasmic reticulum, and are involved in neuroendocrine secretion or in membrane trafficking in neuroendocrine cells. The product of this gene is a potent neurite outgrowth inhibitor that may also help block the regeneration of the central nervous system in higher vertebrates. Alternatively spliced transcript variants derived both from differential splicing and differential promoter usage and encoding different isoforms have been identified. There are three isoform ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neuroregeneration
Neuroregeneration is the regrowth or repair of nervous tissues, cells or cell products. Neuroregenerative mechanisms may include generation of new neurons, glia, axons, myelin, or synapses. Neuroregeneration differs between the peripheral nervous system (PNS) and the central nervous system (CNS) by the functional mechanisms involved, especially in the extent and speed of repair. When an axon is damaged, the distal segment undergoes Wallerian degeneration, losing its myelin sheath. The proximal segment can either die by apoptosis or undergo the chromatolytic reaction, which is an attempt at repair. In the CNS, synaptic stripping occurs as glial foot processes invade the dead synapse. Nervous system injuries affect over 90,000 people every year. Spinal cord injuries alone affect an estimated 10,000 people each year. As a result of this high incidence of neurological injuries, nerve regeneration and repair, a subfield of neural tissue engineering, is becoming a rapidly growing ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Central Nervous System
The central nervous system (CNS) is the part of the nervous system consisting primarily of the brain, spinal cord and retina. The CNS is so named because the brain integrates the received information and coordinates and influences the activity of all parts of the bodies of bilateria, bilaterally symmetric and triploblastic animals—that is, all multicellular animals except sponges and Coelenterata, diploblasts. It is a structure composed of nervous tissue positioned along the Anatomical_terms_of_location#Rostral,_cranial,_and_caudal, rostral (nose end) to caudal (tail end) axis of the body and may have an enlarged section at the rostral end which is a brain. Only arthropods, cephalopods and vertebrates have a true brain, though precursor structures exist in onychophorans, gastropods and lancelets. The rest of this article exclusively discusses the vertebrate central nervous system, which is radically distinct from all other animals. Overview In vertebrates, the brain and spinal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycoprotein
Glycoproteins are proteins which contain oligosaccharide (sugar) chains covalently attached to amino acid side-chains. The carbohydrate is attached to the protein in a cotranslational or posttranslational modification. This process is known as glycosylation. Secreted extracellular proteins are often glycosylated. In proteins that have segments extending extracellularly, the extracellular segments are also often glycosylated. Glycoproteins are also often important integral membrane proteins, where they play a role in cell–cell interactions. It is important to distinguish endoplasmic reticulum-based glycosylation of the secretory system from reversible cytosolic-nuclear glycosylation. Glycoproteins of the cytosol and nucleus can be modified through the reversible addition of a single GlcNAc residue that is considered reciprocal to phosphorylation and the functions of these are likely to be an additional regulatory mechanism that controls phosphorylation-based signalling. In ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |