|
Morphinans
Morphinan is the prototype chemical structure of a large chemical class of psychoactive drugs, consisting of opiate analgesics, cough suppressants, and dissociative drug, dissociative hallucinogens, among others. Typical examples include compounds such as morphine, codeine, and dextromethorphan (DXM). Despite related molecular structures, the pharmacological profiles and mechanisms of action between the various types of morphinan substances can vary substantially. They tend to function either as μ-opioid receptor agonists (analgesics), or NMDA receptor antagonists (dissociatives). Structure Morphinan has a phenanthrene core structure with the ''A'' ring remaining aromatic and the ''B'' and ''C'' rings being saturated, and an additional nitrogen-containing, six-membered, saturated ring, the ''D'' ring, being attached to carbons 9 and 13 of the core, and with the nitrogen being at position 17 of the composite. Of the major naturally occurring opiates of the morphinan type—morp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
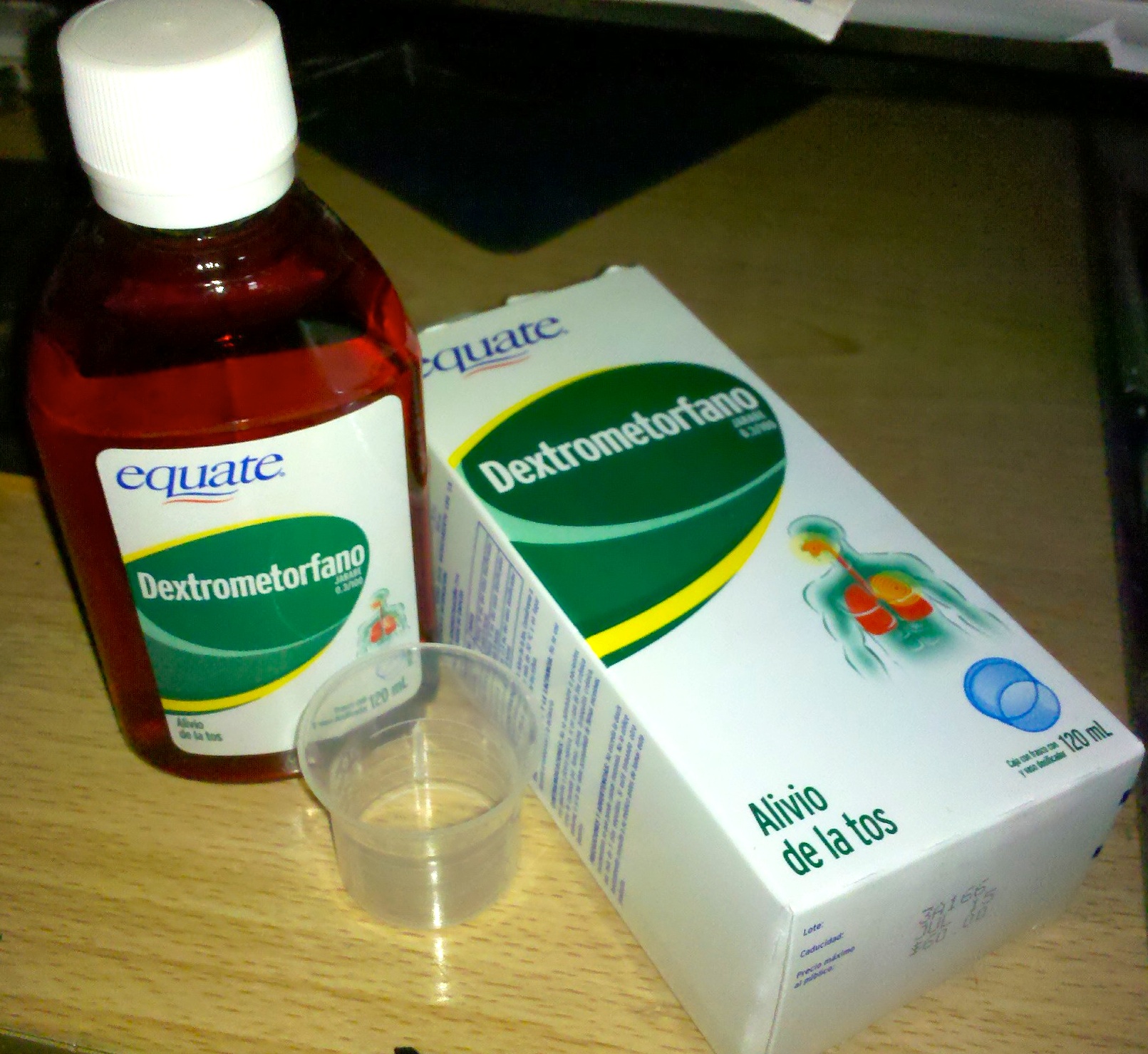
Dextromethorphan
Dextromethorphan, sold under the brand name Robitussin among others, is a cough suppressant used in many cough and Common cold, cold medicines. In 2022, the US Food and Drug Administration (FDA) approved the combination dextromethorphan/bupropion to serve as a rapid-acting antidepressant in people with major depressive disorder. It is in the morphinan class of medications with sedative, dissociative, and stimulant properties (at lower doses). Dextromethorphan does not have a significant Affinity (pharmacology), affinity for the Μ-opioid receptor, mu-opioid receptor activity typical of morphinan compounds and exerts its therapeutic effects through several other receptors. In its pure form, dextromethorphan occurs as a white powder. When exceeding approved dosages, dextromethorphan acts as a dissociative, dissociative hallucinogen. It has multiple mechanism of action, mechanisms of action, including actions as a nonselective serotonin reuptake inhibitor and a sigma-1 receptor ag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Analgesic
An analgesic drug, also called simply an analgesic, antalgic, pain reliever, or painkiller, is any member of the group of drugs used for pain management. Analgesics are conceptually distinct from anesthetics, which temporarily reduce, and in some instances eliminate, sensation, although analgesia and anesthesia are neurophysiologically overlapping and thus various drugs have both analgesic and anesthetic effects. Analgesic choice is also determined by the type of pain: For neuropathic pain, recent research has suggested that classes of drugs that are not normally considered analgesics, such as tricyclic antidepressants and anticonvulsants may be considered as an alternative. Various analgesics, such as many NSAIDs, are available over the counter in most countries, whereas various others are prescription drugs owing to the substantial risks and high chances of overdose, misuse, and addiction in the absence of medical supervision. Etymology The word ''analgesic'' derive ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
International Union Of Pure And Applied Chemistry
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is a member of the International Science Council (ISC). IUPAC is registered in Zürich, Switzerland, and the administrative office, known as the "IUPAC Secretariat", is in Research Triangle Park, North Carolina, United States. IUPAC's executive director heads this administrative office, currently Greta Heydenrych. IUPAC was established in 1919 as the successor of the International Congress of Applied Chemistry for the advancement of chemistry. Its members, the National Adhering Organizations, can be national chemistry societies, national academies of sciences, or other bodies representing chemists. There are fifty-four National Adhering Organizations and three Associate National Adhering Organizations. IUPAC's Inter-divisional Committee ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thebaine
Thebaine (paramorphine), also known as codeine methyl enol ether, is an opiate alkaloid, its name coming from the Greek Θῆβαι, '' Thēbai'' (Thebes), an ancient city in Upper Egypt. A minor constituent of opium, thebaine is chemically similar to both morphine and codeine, but has stimulatory rather than depressant effects. At high doses, it causes convulsions similar to strychnine poisoning. The synthetic enantiomer (+)-thebaine does show analgesic effects apparently mediated through opioid receptors, unlike the inactive natural enantiomer (−)-thebaine. While thebaine is not used therapeutically, it is the main alkaloid extracted from '' Papaver bracteatum'' (Iranian opium / Persian poppy) and can be converted industrially into a variety of compounds, including hydrocodone, hydromorphone, oxycodone, oxymorphone, nalbuphine, naloxone, naltrexone, buprenorphine, butorphanol and etorphine. Thebaine is controlled under international law, is listed as a Class A dr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diprenorphine
Diprenorphine (brand name Revivon; former developmental code name M5050), also known as diprenorfin, is a non-selective, high-affinity, weak partial agonist of the μ- (MOR), κ- (KOR), and δ-opioid receptor (DOR) (with equal affinity) which is used in veterinary medicine as an opioid antagonist. It is used to reverse the effects of super-potent opioid analgesics such as etorphine and carfentanil that are used for tranquilizing large animals. The drug is not approved for use in humans. Diprenorphine is the strongest opioid antagonist that is commercially available (some 100 times more potent than nalorphine), and is used for reversing the effects of very strong opioids for which the binding affinity is so high that naloxone does not effectively or reliably reverse the narcotic effects. These super-potent opioids, with the single exception of buprenorphine (which has an improved safety-profile due to its partial agonism character), are not used in humans because the dose ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Etorphine
Etorphine (M99) is a semi-synthetic opioid possessing an analgesic potency approximately 1,000–3,000 times that of morphine. It was first prepared in 1960 from oripavine, which does not generally occur in opium poppy extract but rather the related plants '' Papaver orientale'' and '' Papaver bracteatum''. It was reproduced in 1963 by a research group at MacFarlan Smith in Edinburgh, led by Kenneth Bentley. It can be produced from thebaine. Veterinary use Etorphine is available legally only for veterinary use and is strictly governed by law. It is often used to immobilise elephants and other large mammals. Diprenorphine (Revivon) is an opioid receptor antagonist that can be administered in proportion to the amount of etorphine used (1.3 times) to reverse its effects. Veterinary-strength etorphine is fatal to humans. For this reason the package as supplied to vets always includes the human antidote along with the etorphine. The human antidote is generally naloxone, not dipr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Naloxegol
Naloxegol ( INN; PEGylated naloxol; trade names Movantik and Moventig) is a peripherally acting μ-opioid receptor antagonist developed by AstraZeneca, licensed from Nektar Therapeutics, for the treatment of opioid-induced constipation. It was approved in 2014 in adult patients with chronic, non-cancer pain. Doses of 25 mg were found safe and well tolerated for 52 weeks. When given concomitantly with opioid analgesics, naloxegol reduced constipation-related side effects, while maintaining comparable levels of analgesia. The most common side effects are abdominal pain, diarrhea, nausea, flatulence, vomiting, and headache. Naloxegol was previously a Schedule II drug in the United States because of its chemical similarity to opium alkaloids. It was officially decontrolled in January 2015. It was reclassified as a prescription drug after the FDA and DEA concluded that the impermeability of the blood–brain barrier to this compound made it non-habit-forming, and so witho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
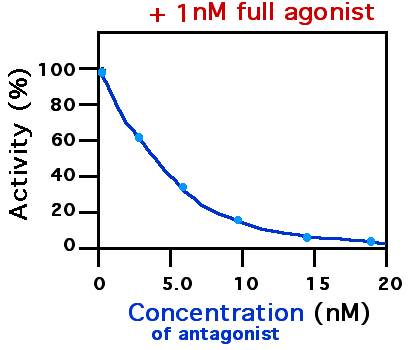
Receptor Antagonist
A receptor antagonist is a type of receptor ligand or drug that blocks or dampens a biological response by binding to and blocking a receptor rather than activating it like an agonist. Antagonist drugs interfere in the natural operation of receptor proteins.Pharmacology Guide: In vitro pharmacology: concentration-response curves ." '' GlaxoWellcome.'' Retrieved on December 6, 2007. They are sometimes called blockers; examples include alpha blockers, beta b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Agonist
An agonist is a chemical that activates a Receptor (biochemistry), receptor to produce a biological response. Receptors are Cell (biology), cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an Receptor antagonist, antagonist blocks the action of the agonist, while an inverse agonist causes an action opposite to that of the agonist. Etymology The word originates from the Ancient Greek, Greek word (''agōnistēs''), "contestant; champion; rival" < (''agōn''), "contest, combat; exertion, struggle" < (''agō''), "I lead, lead towards, conduct; drive." Types of agonists Receptor (biochemistry), Receptors can be activated by either endogenous agonists (such as hormones and neurotransmitters) or exogenous agonists (such as medication, drugs), resulting in a biological response. A physiological agonism an ...[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Receptor Antagonist
A receptor antagonist is a type of receptor ligand or drug that blocks or dampens a biological response by binding to and blocking a receptor rather than activating it like an agonist. Antagonist drugs interfere in the natural operation of receptor proteins.Pharmacology Guide: In vitro pharmacology: concentration-response curves ." '' GlaxoWellcome.'' Retrieved on December 6, 2007. They are sometimes called blockers; examples include alpha blockers, beta b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Levorotatory
Optical rotation, also known as polarization rotation or circular birefringence, is the rotation of the orientation of the plane of polarization about the optical axis of linearly polarized light as it travels through certain materials. Circular birefringence and circular dichroism are the manifestations of optical activity. Optical activity occurs only in chiral materials, those lacking microscopic mirror symmetry. Unlike other sources of birefringence which alter a beam's state of polarization, optical activity can be observed in fluids. This can include gases or solutions of chiral molecules such as sugars, molecules with helical secondary structure such as some proteins, and also chiral liquid crystals. It can also be observed in chiral solids such as certain crystals with a rotation between adjacent crystal planes (such as quartz) or metamaterials. When looking at the source of light, the rotation of the plane of polarization may be either to the right (dextrorotatory o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
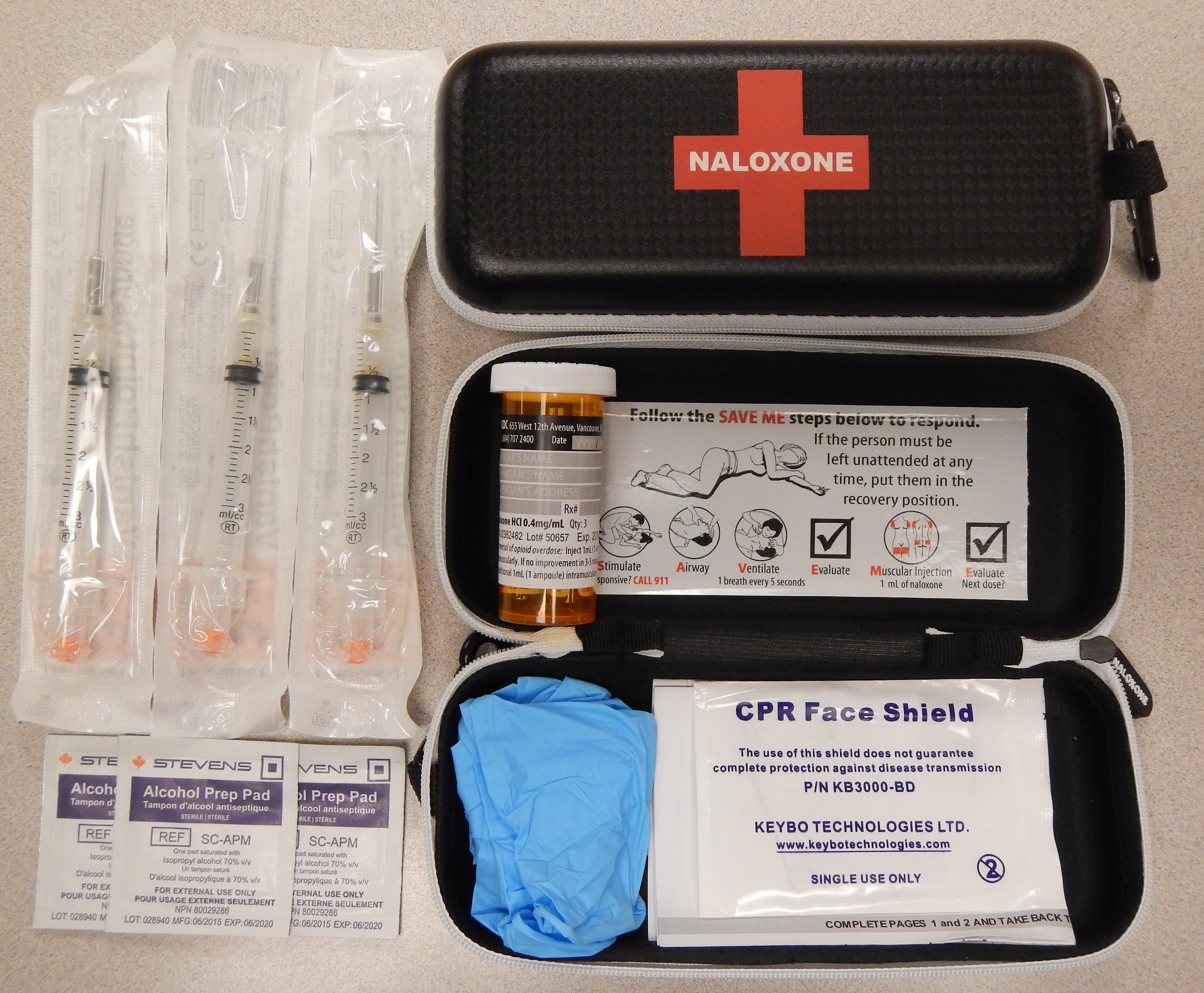
Naloxone
Naloxone, sold under the brand name Narcan among others, is an opioid antagonist, a medication used to reverse or reduce the effects of opioids. For example, it is used to restore breathing after an opioid overdose. Effects begin within two minutes when given intravenously, five minutes when injected into a muscle, and ten minutes as a nasal spray. Naloxone blocks the effects of opioids for 30 to 90 minutes. Administration to opioid-dependent individuals may cause symptoms of opioid withdrawal, including restlessness, agitation, nausea, vomiting, a fast heart rate, and sweating. To prevent this, small doses every few minutes can be given until the desired effect is reached. In those with previous heart disease or taking medications that negatively affect the heart, further heart problems have occurred. It appears to be safe in pregnancy, after having been given to a limited number of women. Naloxone is a non-selective and competitive opioid receptor antagonist. It revers ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |