|
Molten Metal
Melting, or Enthalpy of fusion, fusion, is a physical process that results in the phase transition of a chemical substance, substance from a solid to a liquid. This occurs when the internal energy of the solid increases, typically by the application of heat or Divorce, pressure, which increases the substance's temperature to the melting point. At the melting point, the ordering of ions or molecules in the solid breaks down to a less ordered state, and the solid melts to become a liquid. Substances in the molten state generally have reduced viscosity as the temperature increases. An exception to this principle is Allotropes of sulfur, elemental sulfur, whose viscosity increases in the range of 130 °C to 190 °C due to polymerization. Some organic compounds melt through mesophases, states of partial order between solid and liquid. First order phase transition From a thermodynamics point of view, at the melting point the change in Gibbs free energy ''∆G'' of the s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamics
Thermodynamics is a branch of physics that deals with heat, Work (thermodynamics), work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of thermodynamics, which convey a quantitative description using measurable macroscopic physical quantity, physical quantities but may be explained in terms of microscopic constituents by statistical mechanics. Thermodynamics applies to various topics in science and engineering, especially physical chemistry, biochemistry, chemical engineering, and mechanical engineering, as well as other complex fields such as meteorology. Historically, thermodynamics developed out of a desire to increase the thermodynamic efficiency, efficiency of early steam engines, particularly through the work of French physicist Nicolas Léonard Sadi Carnot, Sadi Carnot (1824) who believed that engine efficiency was the key that could help France win ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macroscopic single crystals are usually identifiable by their geometrical shape, consisting of flat faces with specific, characteristic orientations. The scientific study of crystals and crystal formation is known as crystallography. The process of crystal formation via mechanisms of crystal growth is called crystallization or solidification. The word ''crystal'' derives from the Ancient Greek word (), meaning both "ice" and " rock crystal", from (), "icy cold, frost". Examples of large crystals include snowflakes, diamonds, and table salt. Most inorganic solids are not crystals but polycrystals, i.e. many microscopic crystals fused together into a single solid. Polycrystals include most metals, rocks, ceramics, and ice. A third cat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
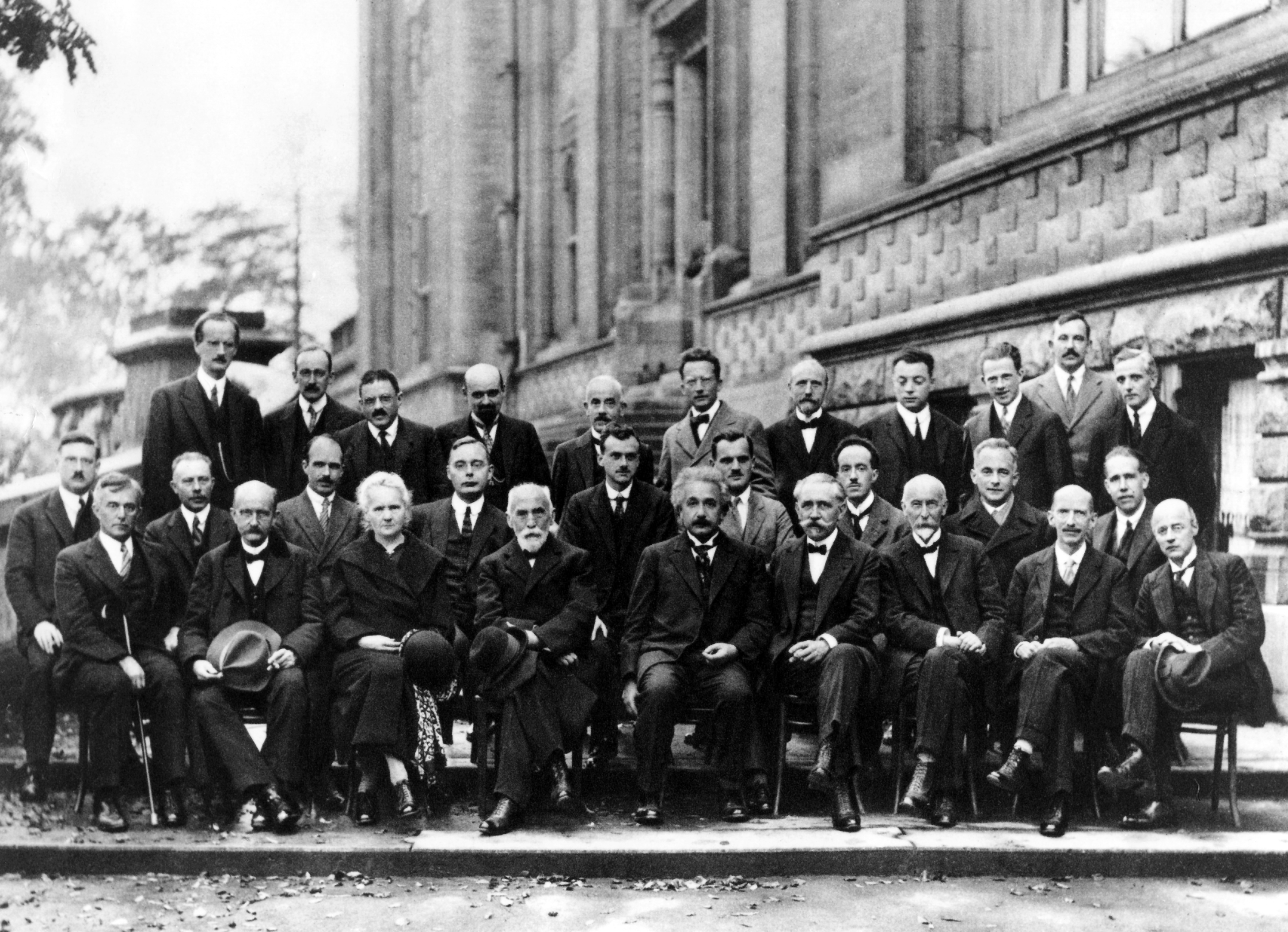
Max Born
Max Born (; 11 December 1882 – 5 January 1970) was a German-British theoretical physicist who was instrumental in the development of quantum mechanics. He also made contributions to solid-state physics and optics, and supervised the work of a number of notable physicists in the 1920s and 1930s. Born shared the 1954 Nobel Prize in Physics with Walther Bothe "for his fundamental research in quantum mechanics, especially in the statistical interpretation of the wave function". Born entered the University of Göttingen in 1904, where he met the three renowned mathematicians Felix Klein, David Hilbert, and Hermann Minkowski. He wrote his PhD thesis on the subject of the stability of elastic wires and tapes, winning the university's Philosophy Faculty Prize. In 1905, he began researching special relativity with Minkowski, and subsequently wrote his habilitation thesis on the Thomson model of the atom. A chance meeting with Fritz Haber in Berlin in 1918 led to discussion of how an io ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Frederick Lindemann, 1st Viscount Cherwell
Frederick Alexander Lindemann, 1st Viscount Cherwell, ( ; 5 April 18863 July 1957) was a British physicist who was prime scientific adviser to Winston Churchill in World War II. He was involved in the development of radar and infra-red guidance systems. He was sceptical of the first reports of the enemy's V-weapons programme. He pressed the case for the strategic area bombing of cities. His abiding influence on Churchill stemmed from close personal friendship, as a member of the latter's country-house set. In Churchill's second government, he was given a seat in the cabinet, and later created Viscount Cherwell of Oxford. Early life, family and personality Lindemann was the second of three sons of Adolph Friedrich Lindemann, who had emigrated to the United Kingdom circa 1871 and became naturalised. – See especially p. 343. Frederick was born in Baden-Baden in Germany, where his American mother Olga Noble, the widow of a wealthy banker, was taking "the cure". After scho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helium-4
Helium-4 () is a stable isotope of the element helium. It is by far the more abundant of the two naturally occurring isotopes of helium, making up about 99.99986% of the helium on Earth. Its nucleus is identical to an alpha particle, and consists of two protons and two neutrons. Helium-4 makes up about one quarter of the ordinary matter in the universe by mass, with almost all of the rest being hydrogen. While nuclear fusion in stars also produces helium-4, most of the helium-4 in the Sun and in the universe is thought to have been produced during the Big Bang, known as " primordial helium". However, primordial helium-4 is largely absent from the Earth, having escaped during the high-temperature phase of Earth's formation. On Earth, most naturally occurring helium-4 is produced by the alpha decay of heavy elements in the Earth's crust, after the planet cooled and solidified. When liquid helium-4 is cooled to below , it becomes a superfluid, with properties very different from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helium-3
Helium-3 (3He see also helion) is a light, stable isotope of helium with two protons and one neutron. (In contrast, the most common isotope, helium-4, has two protons and two neutrons.) Helium-3 and hydrogen-1 are the only stable nuclides with more protons than neutrons. It was discovered in 1939. Helium-3 atoms are fermionic and become a superfluid at the temperature of 2.491 mK. Helium-3 occurs as a primordial nuclide, escaping from Earth's crust into its atmosphere and into outer space over millions of years. It is also thought to be a natural nucleogenic and cosmogenic nuclide, one produced when lithium is bombarded by natural neutrons, which can be released by spontaneous fission and by nuclear reactions with cosmic rays. Some found in the terrestrial atmosphere is a remnant of atmospheric and underwater nuclear weapons testing. Nuclear fusion using helium-3 has long been viewed as a desirable future energy source. The fusion of two of its atoms would be aneut ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helium
Helium (from ) is a chemical element; it has chemical symbol, symbol He and atomic number 2. It is a colorless, odorless, non-toxic, inert gas, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling point is the lowest among all the Chemical element, elements, and it does not have a melting point at standard pressures. It is the second-lightest and second-most Abundance of the chemical elements, abundant element in the observable universe, after hydrogen. It is present at about 24% of the total elemental mass, which is more than 12 times the mass of all the heavier elements combined. Its abundance is similar to this in both the Sun and Jupiter, because of the very high nuclear binding energy (per nucleon) of helium-4 with respect to the next three elements after helium. This helium-4 binding energy also accounts for why it is a product of both nuclear fusion and radioactive decay. The most common isotope of helium in the universe is helium-4, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase Transition
In physics, chemistry, and other related fields like biology, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic State of matter, states of matter: solid, liquid, and gas, and in rare cases, plasma (physics), plasma. A phase of a thermodynamic system and the states of matter have uniform physical property, physical properties. During a phase transition of a given medium, certain properties of the medium change as a result of the change of external conditions, such as temperature or pressure. This can be a discontinuous change; for example, a liquid may become gas upon heating to its boiling point, resulting in an abrupt change in volume. The identification of the external conditions at which a transformation occurs defines the phase transition point. Types of phase transition States of matter Phase transitions commonly refer to when a substance tran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Entropy Of Fusion
In thermodynamics, the entropy of fusion is the increase in entropy when melting a solid substance. This is almost always positive since the degree of disorder increases in the transition from an organized crystalline solid to the disorganized structure of a liquid; the only known exception is helium. It is denoted as \Delta S_ and normally expressed in joules per mole-kelvin, J/(mol·K). A natural process such as a phase transition will occur when the associated change in the Gibbs free energy is negative. :\Delta G_ = \Delta H_ - T \times \Delta S_ < 0, where is the enthalpy of fusion. Since this is a thermodynamic equation, the symbol refers to the absolute , measured in |
Latent Heat
Latent heat (also known as latent energy or heat of transformation) is energy released or absorbed, by a body or a thermodynamic system, during a constant-temperature process—usually a first-order phase transition, like melting or condensation. Latent heat can be understood as hidden energy which is supplied or extracted to change the state of a substance without changing its temperature or pressure. This includes the latent heat of fusion (solid to liquid), the latent heat of vaporization (liquid to gas) and the latent heat of sublimation (solid to gas). The term was introduced around 1762 by Scottish chemist Joseph Black. Black used the term in the context of calorimetry where a heat transfer caused a volume change in a body while its temperature was constant. In contrast to latent heat, sensible heat is energy transferred as heat, with a resultant temperature change in a body. Usage The terms ''sensible heat'' and ''latent heat'' refer to energy transferred between a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enthalpy Of Fusion
In thermodynamics, the enthalpy of fusion of a substance, also known as (latent) heat of fusion, is the change in its enthalpy resulting from providing energy, typically heat, to a specific quantity of the substance to change its state from a solid to a liquid, at constant pressure. The enthalpy of fusion is the amount of energy required to convert one mole of solid into liquid. For example, when melting 1 kg of ice (at 0 °C under a wide range of pressures), 333.55 kJ of energy is absorbed with no temperature change. The heat of solidification (when a substance changes from liquid to solid) is equal and opposite. This energy includes the contribution required to make room for any associated change in volume by displacing its environment against ambient pressure. The temperature at which the phase transition occurs is the melting point or the freezing point, according to context. By convention, the pressure is assumed to be unless otherwise specified. Overview T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |