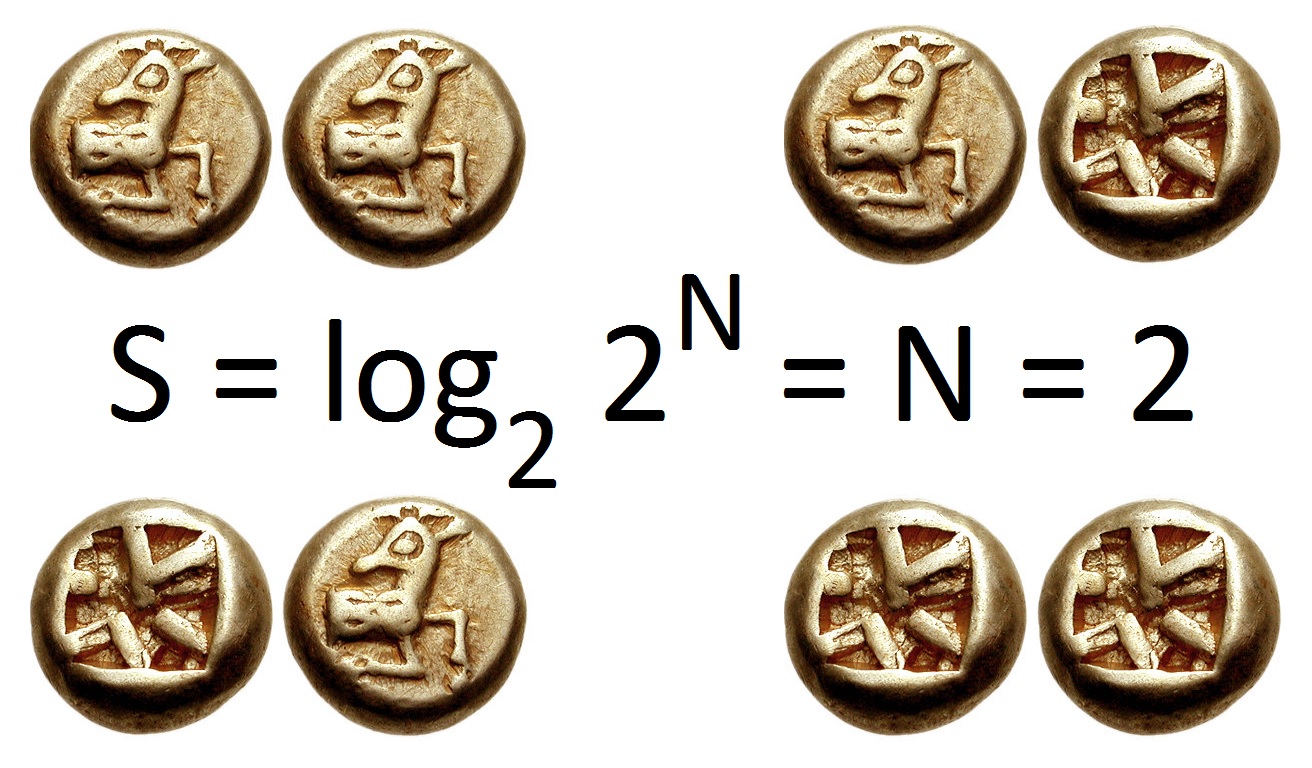|
Minimum Efficiency Performance Standards
A minimum energy performance standard (MEPS) is a specification, containing a number of performance requirements for an energy-using device, that effectively limits the maximum amount of energy that may be consumed by a product in performing a specified task. An MEPS is usually made mandatory by a government's Efficient energy use, energy efficiency body. It may include requirements not directly related to energy; this is to ensure that general performance and user satisfaction are not adversely affected by increasing energy efficiency. It generally requires use of a particular test procedure that specifies how performance is measured. In North America when addressing energy efficiency, a MEPS is sometimes referred to simply as a "standard", as in "Co-operation on Collaborative Labeling and Appliance Standards Program (CLASP), Labeling and Standards Programs". In Latin America when addressing energy efficiency, MEPS are sometimes referred to as ''Normas'' (translated as "norms"). ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Energy
Energy () is the physical quantity, quantitative physical property, property that is transferred to a physical body, body or to a physical system, recognizable in the performance of Work (thermodynamics), work and in the form of heat and light. Energy is a Conservation law, conserved quantity—the law of conservation of energy states that energy can be Energy transformation, converted in form, but not created or destroyed. The unit of measurement for energy in the International System of Units (SI) is the joule (J). Forms of energy include the kinetic energy of a moving object, the potential energy stored by an object (for instance due to its position in a Classical field theory, field), the elastic energy stored in a solid object, chemical energy associated with chemical reactions, the radiant energy carried by electromagnetic radiation, the internal energy contained within a thermodynamic system, and rest energy associated with an object's rest mass. These are not mutual ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Expected Value
In probability theory, the expected value (also called expectation, expectancy, expectation operator, mathematical expectation, mean, expectation value, or first Moment (mathematics), moment) is a generalization of the weighted average. Informally, the expected value is the arithmetic mean, mean of the possible values a random variable can take, weighted by the probability of those outcomes. Since it is obtained through arithmetic, the expected value sometimes may not even be included in the sample data set; it is not the value you would expect to get in reality. The expected value of a random variable with a finite number of outcomes is a weighted average of all possible outcomes. In the case of a continuum of possible outcomes, the expectation is defined by Integral, integration. In the axiomatic foundation for probability provided by measure theory, the expectation is given by Lebesgue integration. The expected value of a random variable is often denoted by , , or , with a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Onsager Reciprocal Relations
In thermodynamics, the Onsager reciprocal relations express the equality of certain ratios between flows and forces in thermodynamic systems out of equilibrium, but where a notion of local equilibrium exists. "Reciprocal relations" occur between different pairs of forces and flows in a variety of physical systems. For example, consider fluid systems described in terms of temperature, matter density, and pressure. In this class of systems, it is known that temperature differences lead to heat flows from the warmer to the colder parts of the system; similarly, pressure differences will lead to matter flow from high-pressure to low-pressure regions. What is remarkable is the observation that, when both pressure and temperature vary, temperature differences at constant pressure can cause matter flow (as in convection) and pressure differences at constant temperature can cause heat flow. Perhaps surprisingly, the heat flow per unit of pressure difference and the density (matter) flo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Non-equilibrium Thermodynamics
Non-equilibrium thermodynamics is a branch of thermodynamics that deals with physical systems that are not in thermodynamic equilibrium but can be described in terms of macroscopic quantities (non-equilibrium state variables) that represent an extrapolation of the variables used to specify the system in thermodynamic equilibrium. Non-equilibrium thermodynamics is concerned with transport processes and with the rates of chemical reactions. Almost all systems found in nature are not in thermodynamic equilibrium, for they are changing or can be triggered to change over time, and are continuously and discontinuously subject to flux of matter and energy to and from other systems and to chemical reactions. Many systems and processes can, however, be considered to be in equilibrium locally, thus allowing description by currently known equilibrium thermodynamics. Nevertheless, some natural systems and processes remain beyond the scope of equilibrium thermodynamic methods due to the exis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MaxEnt School
In physics, maximum entropy thermodynamics (colloquially, ''MaxEnt'' thermodynamics) views equilibrium thermodynamics and statistical mechanics as inference processes. More specifically, MaxEnt applies inference techniques rooted in Shannon information theory, Bayesian probability, and the principle of maximum entropy. These techniques are relevant to any situation requiring prediction from incomplete or insufficient data (e.g., image reconstruction, signal processing, spectral analysis, and inverse problems). MaxEnt thermodynamics began with two papers by Edwin T. Jaynes published in the 1957 ''Physical Review''. Maximum Shannon entropy Central to the MaxEnt thesis is the principle of maximum entropy. It demands as given some partly specified model and some specified data related to the model. It selects a preferred probability distribution to represent the model. The given data state "testable information" about the probability distribution, for example particular expec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clausius
Rudolf Julius Emanuel Clausius (; 2 January 1822 – 24 August 1888) was a German physicist and mathematician and is considered one of the central founding fathers of the science of thermodynamics. By his restatement of Nicolas Léonard Sadi Carnot, Sadi Carnot's principle known as the Carnot cycle, he gave the theory of heat a truer and sounder basis. His most important paper, "On the Moving Force of Heat", published in 1850, first stated the basic ideas of the second law of thermodynamics. In 1865 he introduced the concept of entropy. In 1870 he introduced the virial theorem, which applied to heat. Life Clausius was born in Koszalin, Köslin (now Koszalin, Poland) in the Province of Pomerania (1815–1945), Province of Pomerania in Prussia. His father was a Protestant pastor and school inspector, and Rudolf studied in the school of his father. In 1838, he went to the Gymnasium (school), Gymnasium in Szczecin, Stettin. Clausius graduated from the University of Berlin in 1844 wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boltzmann Constant
The Boltzmann constant ( or ) is the proportionality factor that relates the average relative thermal energy of particles in a ideal gas, gas with the thermodynamic temperature of the gas. It occurs in the definitions of the kelvin (K) and the molar gas constant, in Planck's law of black-body radiation and Boltzmann's entropy formula, and is used in calculating Johnson–Nyquist noise, thermal noise in resistors. The Boltzmann constant has Dimensional analysis, dimensions of energy divided by temperature, the same as entropy and heat capacity. It is named after the Austrian scientist Ludwig Boltzmann. As part of the 2019 revision of the SI, the Boltzmann constant is one of the seven "Physical constant, defining constants" that have been defined so as to have exact finite decimal values in SI units. They are used in various combinations to define the seven SI base units. The Boltzmann constant is defined to be exactly joules per kelvin, with the effect of defining the SI unit ke ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Information Entropy
In information theory, the entropy of a random variable quantifies the average level of uncertainty or information associated with the variable's potential states or possible outcomes. This measures the expected amount of information needed to describe the state of the variable, considering the distribution of probabilities across all potential states. Given a discrete random variable X, which may be any member x within the set \mathcal and is distributed according to p\colon \mathcal\to , 1/math>, the entropy is \Eta(X) := -\sum_ p(x) \log p(x), where \Sigma denotes the sum over the variable's possible values. The choice of base for \log, the logarithm, varies for different applications. Base 2 gives the unit of bits (or " shannons"), while base ''e'' gives "natural units" nat, and base 10 gives units of "dits", "bans", or " hartleys". An equivalent definition of entropy is the expected value of the self-information of a variable. The concept of information entropy was ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
State Function
In the thermodynamics of equilibrium, a state function, function of state, or point function for a thermodynamic system is a mathematical function relating several state variables or state quantities (that describe equilibrium states of a system) that depend only on the current equilibrium thermodynamic state of the system (e.g. gas, liquid, solid, crystal, or emulsion), not the path which the system has taken to reach that state. A state function describes equilibrium states of a system, thus also describing the type of system. A state variable is typically a state function so the determination of other state variable values at an equilibrium state also determines the value of the state variable as the state function at that state. The ideal gas law is a good example. In this law, one state variable (e.g., pressure, volume, temperature, or the amount of substance in a gaseous equilibrium system) is a function of other state variables so is regarded as a state function. A stat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Entropy (classical Thermodynamics)
In classical thermodynamics, entropy () is a property of a thermodynamic system that expresses the direction or outcome of spontaneous changes in the system. The term was introduced by Rudolf Clausius in the mid-19th century to explain the relationship of the internal energy that is available or unavailable for transformations in form of heat and work. Entropy predicts that certain processes are irreversible or impossible, despite not violating the conservation of energy. The definition of entropy is central to the establishment of the second law of thermodynamics, which states that the entropy of isolated systems cannot decrease with time, as they always tend to arrive at a state of thermodynamic equilibrium, where the entropy is highest. Entropy is therefore also considered to be a measure of disorder in the system. Ludwig Boltzmann explained the entropy as a measure of the number of possible microscopic configurations of the individual atoms and molecules of the system (micr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Partition Function (statistical Mechanics)
In physics, a partition function describes the statistics, statistical properties of a system in thermodynamic equilibrium. Partition functions are function (mathematics), functions of the thermodynamic state function, state variables, such as the temperature and volume. Most of the aggregate thermodynamics, thermodynamic variables of the system, such as the energy, total energy, Thermodynamic free energy, free energy, entropy, and pressure, can be expressed in terms of the partition function or its derivatives. The partition function is dimensionless. Each partition function is constructed to represent a particular statistical ensemble (which, in turn, corresponds to a particular Thermodynamic free energy, free energy). The most common statistical ensembles have named partition functions. The canonical partition function applies to a canonical ensemble, in which the system is allowed to exchange heat with the Environment (systems), environment at fixed temperature, volume, an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |