|
Microbiologically Induced Calcite Precipitation
Microbiologically induced calcium carbonate precipitation (MICP) is a bio-geochemical process that induces calcium carbonate precipitation within the soil matrix. Biomineralization in the form of calcium carbonate precipitation can be traced back to the Precambrian period. Calcium carbonate can be precipitated in three polymorphic forms, which in the order of their usual stabilities are calcite, aragonite and vaterite. The main groups of microorganisms that can induce the carbonate precipitation are photosynthetic microorganisms such as cyanobacteria and microalgae; sulfate-reducing bacteria; and some species of microorganisms involved in nitrogen cycle. Several mechanisms have been identified by which bacteria can induce the calcium carbonate precipitation, including urea hydrolysis, denitrification, sulfate production, and iron reduction. Two different pathways, or autotrophic and heterotrophic pathways, through which calcium carbonate is produced have been identified. There are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Carbonate
Calcium carbonate is a chemical compound with the chemical formula . It is a common substance found in Rock (geology), rocks as the minerals calcite and aragonite, most notably in chalk and limestone, eggshells, gastropod shells, shellfish skeletons and pearls. Materials containing much calcium carbonate or resembling it are described as calcareous. Calcium carbonate is the active ingredient in agricultural lime and is produced when calcium ions in hard water react with carbonate ions to form limescale. It has medical use as a calcium supplement or as an antacid, but excessive consumption can be hazardous and cause hypercalcemia and digestive issues. Chemistry Calcium carbonate shares the typical properties of other carbonates. Notably, it: *reacts with acids, releasing carbonic acid which quickly disintegrates into carbon dioxide and water: : *releases carbon dioxide upon heating, called a thermal decomposition reaction, or calcination (to above 840 °C in the case of ), t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Archaebacteria
Archaea ( ) is a domain of organisms. Traditionally, Archaea only included its prokaryotic members, but this has since been found to be paraphyletic, as eukaryotes are known to have evolved from archaea. Even though the domain Archaea cladistically includes eukaryotes, the term "archaea" (: archaeon , from the Greek "ἀρχαῖον", which means ancient) in English still generally refers specifically to prokaryotic members of Archaea. Archaea were initially classified as bacteria, receiving the name archaebacteria (, in the Archaebacteria kingdom), but this term has fallen out of use. Archaeal cells have unique properties separating them from Bacteria and Eukaryota. Archaea are further divided into multiple recognized phyla. Classification is difficult because most have not been isolated in a laboratory and have been detected only by their gene sequences in environmental samples. It is unknown if they can produce endospores. Archaea are often similar to bacteria in siz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chelation
Chelation () is a type of bonding of ions and their molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These ligands are called chelants, chelators, chelating agents, or sequestering agents. They are usually organic compounds, but this is not a necessity. The word ''chelation'' is derived from Greek χηλή, ''chēlē'', meaning "claw"; the ligands lie around the central atom like the claws of a crab. The term ''chelate'' () was first applied in 1920 by Sir Gilbert T. Morgan and H. D. K. Drew, who stated: "The adjective chelate, derived from the great claw or ''chele'' (Greek) of the crab or other crustaceans, is suggested for the caliperlike groups which function as two associating units and fasten to the central atom so as to produce heterocyclic rings." Chelation is useful in applications such as providing nutritional supplements, in chel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lead
Lead () is a chemical element; it has Chemical symbol, symbol Pb (from Latin ) and atomic number 82. It is a Heavy metal (elements), heavy metal that is density, denser than most common materials. Lead is Mohs scale, soft and Ductility, malleable, and also has a relatively low melting point. When freshly cut, lead is a shiny gray with a hint of blue. It tarnishes to a dull gray color when exposed to air. Lead has the highest atomic number of any stable nuclide, stable element and three of its isotopes are endpoints of major nuclear decay chains of heavier elements. Lead is a relatively unreactive post-transition metal. Its weak metallic character is illustrated by its Amphoterism, amphoteric nature; lead and lead oxides react with acids and base (chemistry), bases, and it tends to form covalent bonds. Lead compounds, Compounds of lead are usually found in the +2 oxidation state rather than the +4 state common with lighter members of the carbon group. Exceptions are mostly limited ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Soil Liquefaction
Soil liquefaction occurs when a cohesionless saturated or partially saturated soil substantially loses Shear strength (soil), strength and stiffness in response to an applied Shear stress, stress such as shaking during an earthquake or other sudden change in stress condition, in which material that is ordinarily a solid behaves like a liquid. In soil mechanics, the term "liquefied" was first used by Allen Hazen in reference to the 1918 failure of the Calaveras Dam in California. He described the mechanism of flow liquefaction of the embankment dam as: The phenomenon is most often observed in saturated, loose (low density or uncompacted), sandy soils. This is because a loose sand has a tendency to Compressibility, compress when a force, load is applied. Dense sands, by contrast, tend to expand in volume or 'Reynolds' dilatancy, dilate'. If the soil is saturated by water, a condition that often exists when the soil is below the water table or sea level, then water fills the gap ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cementation (geology)
Minerals bond grains of sediment together by growing around them. This process is called cementation and is a part of the rock cycle. Cementation involves ions carried in groundwater chemically precipitating to form new crystalline material between sedimentary grains. The new pore-filling minerals form "bridges" between original sediment grains, thereby binding them together. In this way, ''sand'' becomes sandstone, and ''gravel'' becomes conglomerate or breccia. Cementation occurs as part of the diagenesis or lithification of sediments. Cementation occurs primarily below the water table regardless of sedimentary grain sizes present. Large volumes of pore water must pass through sediment pores for new mineral cements to crystallize and so millions of years are generally required to complete the cementation process. Common mineral cements include calcite, quartz, and silica phases like cristobalite, iron oxides, and clay minerals; other mineral cements also occur. Cement ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
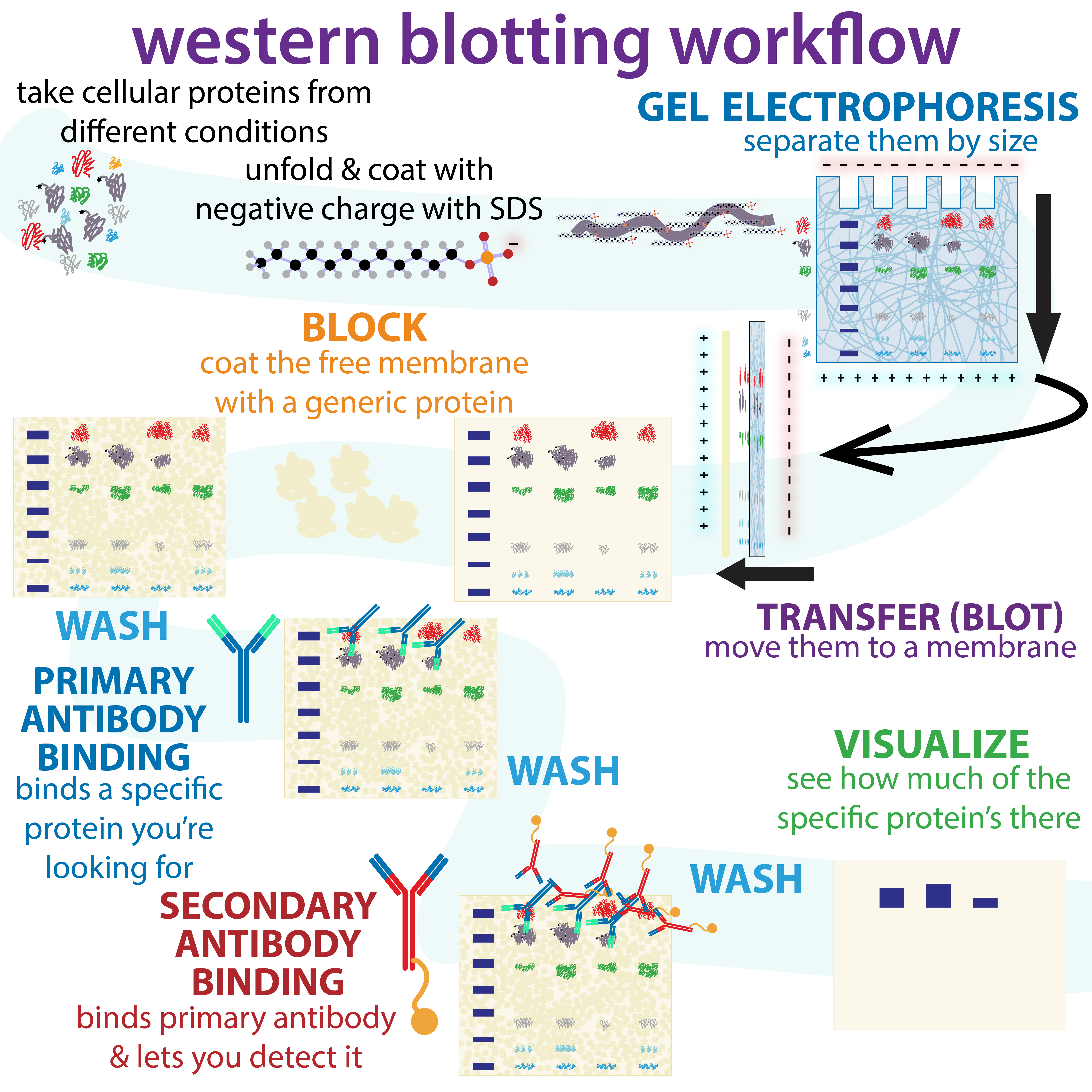
Western Blot
The western blot (sometimes called the protein immunoblot), or western blotting, is a widely used analytical technique in molecular biology and immunogenetics to detect specific proteins in a sample of tissue homogenate or extract. Besides detecting the proteins, this technique is also utilized to visualize, distinguish, and quantify the different proteins in a complicated protein combination. Western blot technique uses three elements to achieve its task of separating a specific protein from a complex: separation by size, transfer of protein to a solid support, and marking target protein using a primary and secondary antibody to visualize. A synthetic or animal-derived antibody (known as the primary antibody) is created that recognizes and binds to a specific target protein. The electrophoresis membrane is washed in a solution containing the primary antibody, before excess antibody is washed off. A secondary antibody is added which recognizes and binds to the primary antibod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plastics
Plastics are a wide range of synthetic or semisynthetic materials composed primarily of polymers. Their defining characteristic, plasticity, allows them to be molded, extruded, or pressed into a diverse range of solid forms. This adaptability, combined with a wide range of other properties such as low weight, durability, flexibility, chemical resistance, low toxicity, and low-cost production, has led to their widespread use around the world. While most plastics are produced from natural gas and petroleum, a growing minority are produced from renewable resources like polylactic acid. Between 1950 and 2017, 9.2 billion metric tons of plastic are estimated to have been made, with more than half of this amount being produced since 2004. In 2023 alone, preliminary figures indicate that over 400 million metric tons of plastic were produced worldwide. If global trends in plastic demand continue, it is projected that annual global plastic production will exceed 1.3 billion tons ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rubber
Rubber, also called India rubber, latex, Amazonian rubber, ''caucho'', or ''caoutchouc'', as initially produced, consists of polymers of the organic compound isoprene, with minor impurities of other organic compounds. Types of polyisoprene that are used as natural rubbers are classified as elastomers. Currently, rubber is harvested mainly in the form of the latex from the Hevea brasiliensis, Pará rubber tree (''Hevea brasiliensis'') or others. The latex is a sticky, milky and white colloid drawn off by making incisions in the bark and collecting the fluid in vessels in a process called "tapping". Manufacturers refine this latex into the rubber that is ready for commercial processing. Natural rubber is used extensively in many applications and products, either alone or in combination with other materials. In most of its useful forms, it has a large stretch ratio and high resilience and also is buoyant and water-proof. Industrial demand for rubber-like materials began to out ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ginger Krieg Dosier
Ginger Krieg Dosier is an American architect who, in 2010, developed a technique for using microbiologically induced calcite precipitation to manufacture bricks for construction. Dosier's brick-making method consists of filling a rectangular form with sand layered intermittently with a solution containing urea, calcium chloride, and the non-pathogenic bacteria Sporosarcina pasteurii. After several days, the bacteria create a chemical chain reaction producing a mineral that binds the sand together into a brick. Because this process does not involve firing the brick in a kiln as in conventional brick-making, Dosier estimates that her method could reduce carbon emissions Greenhouse gas (GHG) emissions from human activities intensify the greenhouse effect. This contributes to climate change. Carbon dioxide (), from burning fossil fuels such as coal, petroleum, oil, and natural gas, is the main cause of climate chan ... by 800 million tons each year. Dosier is the winner of the 201 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbamic Acid
Carbamic acid, which might also be called aminoformic acid or aminocarboxylic acid, is the chemical compound with the formula . It can be obtained by the reaction of ammonia and carbon dioxide at very low temperatures, which also yields ammonium carbamate . The compound is stable only up to about 250 K (−23 °C); at higher temperatures it decomposes into those two gases. The solid apparently consists of dimer (chemistry), dimers, with the two molecules connected by hydrogen bonds between the two carboxyl groups –COOH.J. B. Bossa, P. Theulé, F. Duvernay, F. Borget and T. Chiavassa (2008): "Carbamic acid and carbamate formation in NH3:CO2 ices – UV irradiation versus thermal processes". ''Astronomy and Astrophysics'', volume 492, issue 3, pages 719-724. Carbamic acid could be seen as both an amine and carboxylic acid, and therefore an amino acid;R. K. Khanna and M. H. Moore (1999): "Carbamic acid: molecular structure and IR spectra". ''Spectrochimica Acta Part A: M ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Urea
Urea, also called carbamide (because it is a diamide of carbonic acid), is an organic compound with chemical formula . This amide has two Amine, amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest amide of carbamic acid. Urea serves an important role in the cellular metabolism of nitrogen-containing compounds by animals and is the main nitrogen-containing substance in the urine of mammals. ''Urea'' is Neo-Latin, , , itself from Proto-Indo-European ''*h₂worsom''. It is a colorless, odorless solid, highly soluble in water, and practically non-toxic ( is 15 g/kg for rats). Dissolved in water, it is neither acidic nor base (chemistry), alkaline. The body uses it in many processes, most notably metabolic waste#Nitrogen wastes, nitrogen excretion. The liver forms it by combining two ammonia molecules () with a carbon dioxide () molecule in the urea cycle. Urea is widely used in fertilizers as a source of nitrogen (N) and is an important ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |