|
MgSO4
Magnesium sulfate or magnesium sulphate (in English-speaking countries other than the US) is a chemical compound, a salt with the formula , consisting of magnesium cations (20.19% by mass) and sulfate anions . It is a white crystalline solid, soluble in water but not in ethanol. Magnesium sulfate is usually encountered in the form of a hydrate , for various values of ''n'' between 1 and 11. The most common is the heptahydrate , known as Epsom salt, which is a household chemical with many traditional uses, including bath salts. The main use of magnesium sulfate is in agriculture, to correct soils deficient in magnesium (an essential plant nutrient because of the role of magnesium in chlorophyll and photosynthesis). The monohydrate is favored for this use; by the mid 1970s, its production was 2.3 million tons per year. The anhydrous form and several hydrates occur in nature as minerals, and the salt is a significant component of the water from some springs. Hydrates Magnesium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
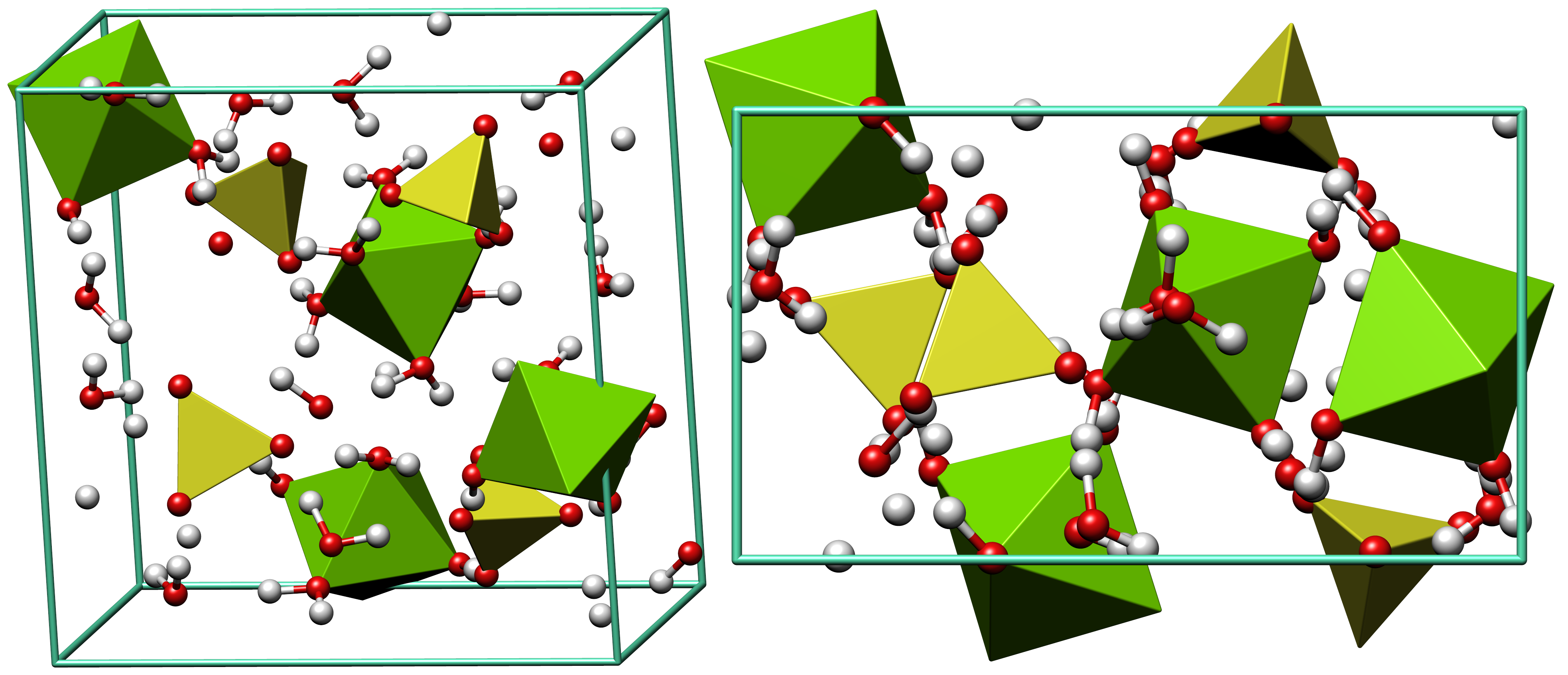
Epsomite
Epsomite, Epsom salt, or magnesium sulfate heptahydrate, is a hydrous magnesium sulfate mineral with formula MgSO4·7H2O. Epsomite crystallizes in the orthorhombic system as rarely found acicular or fibrous crystals, the normal form is as massive encrustations. It is colorless to white with tints of yellow, green and pink. The Mohs hardness is 2 to 2.5 and it has a low specific gravity of 1.67. It is readily soluble in water. It absorbs water from the air and converts to hexahydrate with the loss of one water molecule and a switch to monoclinic structure. Etymology It was first systematically described in 1806 for an occurrence near Epsom, Surrey, England, after which it was named. Discovery and occurrence Epsomite forms as encrustations or efflorescences on limestone cavern walls and mine timbers and walls, rarely as volcanic fumarole deposits, and as rare beds in evaporite layers such as those found in certain bodies of salt water. It occurs in association with melanterit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons while an anion is a negatively charged ion with more electrons than protons. Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed atomic or monatomic ions, while two or more atoms form molecular ions or polyatomic ions. In the case of physical ionization in a fluid (gas or liquid), "ion pairs" are created by spontaneous molecule collisions, where each generated pair consists of a free electron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epsomite
Epsomite, Epsom salt, or magnesium sulfate heptahydrate, is a hydrous magnesium sulfate mineral with formula MgSO4·7H2O. Epsomite crystallizes in the orthorhombic system as rarely found acicular or fibrous crystals, the normal form is as massive encrustations. It is colorless to white with tints of yellow, green and pink. The Mohs hardness is 2 to 2.5 and it has a low specific gravity of 1.67. It is readily soluble in water. It absorbs water from the air and converts to hexahydrate with the loss of one water molecule and a switch to monoclinic structure. Etymology It was first systematically described in 1806 for an occurrence near Epsom, Surrey, England, after which it was named. Discovery and occurrence Epsomite forms as encrustations or efflorescences on limestone cavern walls and mine timbers and walls, rarely as volcanic fumarole deposits, and as rare beds in evaporite layers such as those found in certain bodies of salt water. It occurs in association with melanterit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystallization
Crystallization is the process by which solid forms, where the atoms or molecules are highly organized into a structure known as a crystal. Some ways by which crystals form are precipitating from a solution, freezing, or more rarely deposition directly from a gas. Attributes of the resulting crystal depend largely on factors such as temperature, air pressure, and in the case of liquid crystals, time of fluid evaporation. Crystallization occurs in two major steps. The first is nucleation, the appearance of a crystalline phase from either a supercooled liquid or a supersaturated solvent. The second step is known as crystal growth, which is the increase in the size of particles and leads to a crystal state. An important feature of this step is that loose particles form layers at the crystal's surface and lodge themselves into open inconsistencies such as pores, cracks, etc. The majority of minerals and organic molecules crystallize easily, and the resulting crystals ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spring (water)
A spring is a point of exit at which groundwater from an aquifer flows out on top of Earth's crust (pedosphere) and becomes surface water. It is a component of the hydrosphere. Springs have long been important for humans as a source of fresh water, especially in arid regions which have relatively little annual rainfall. Springs are driven out onto the surface by various natural forces, such as gravity and hydrostatic pressure. Their yield varies widely from a volumetric flow rate of nearly zero to more than for the biggest springs. Formation Springs are formed when groundwater flows onto the surface. This typically happens when the groundwater table reaches above the surface level. Springs may also be formed as a result of karst topography, aquifers, or volcanic activity. Springs also have been observed on the ocean floor, spewing hot water directly into the ocean. Springs formed as a result of karst topography create karst springs, in which ground water travels through ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfate Mineral The sulfate minerals are a class of minerals that include the sulfate ion () within their structure. The sulfate minerals occur commonly in primary evaporite depositional environments, as gangue minerals in hydrothermal veins and as secondary minerals in the oxidizing zone of sulfide mineral deposits. The chromate and manganate minerals have a similar structure and are often included with the sulfates in mineral classification systems.Klein, Cornelis and Cornelius S. Hurlbut, 1985, ''Manual of Mineralogy,'' 20th ed., John Wiley and Sons, New York, pp. 347–354 . Sulfate minerals include: *Anhydrous sulfates ** Barite BaSO4 **Celestite SrSO4 ** Anglesite PbSO4 ** Anhydrite CaSO4 ** Hanksite Na22K(SO4)9(CO3)2Cl *Hydroxide and hydrous sulfates **Gypsum CaSO4·2H2O ** Chalcanthite CuSO4·5H2O ** Kieserite MgS |