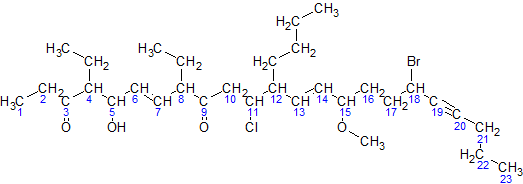
|
Methylpentene
Methylpentene is an alkene with a molecular formula C6H12. The prefix "methyl-" is derived from the fact that there is a methyl(CH3) branch, the word root "-pent-" is derived from the fact that there are 5 carbon atoms in the parent chain, while the " -ene" suffix denotes that there is a double bond present, as per IUPAC nomenclature. Following are the possible structural isomers of methylpentene: See also * Polymethylpentene Polymethylpentene (PMP), also known as poly(4-methyl-1-pentene). It is used for gas-permeable packaging, autoclavable medical and laboratory equipment, microwave components, and cookware. It is commonly called TPX, which is a trademark of Mitsu ... References Alkenes {{hydrocarbon-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C6H12
The molecular formula C6H12 may refer to following structural isomers: Acyclic Compounds *Hexenes ** 1-Hexene ** 2-Hexene ** 3-Hexene * Methylpentenes ** 2-Methyl-1-pentene ** 3-Methyl-1-pentene ** 4-Methyl-1-pentene ** 2-Methyl-2-pentene ** 3-Methyl-2-pentene ** 4-Methyl-2-pentene *Dimethylbutenes **2,3-Dimethyl-1-butene ** 3,3-Dimethyl-1-butene **2,3-Dimethyl-2-butene * 2-Ethyl-1-butene Cyclic compounds * Cyclohexane * Methylcyclopentane * Ethylcyclobutane * Dimethylcyclobutanes ** 1,1-Dimethylcyclobutane ** 1,2-Dimethylcyclobutane ** 1,3-Dimethylcyclobutane * Trimethylcyclopropanes ** 1,1,2-Trimethylcyclopropane ** 1,2,3-Trimethylcyclopropane * Ethylmethylcyclopropanes ** 1-Ethyl-1-methylcyclopropane ** 1-Ethyl-2-methylcyclopropane * Isopropylcyclopropane * Propylcyclopropane ''Note: cis-trans isomers and enantiomers In chemistry, an enantiomer (Help:IPA/English, /ɪˈnænti.əmər, ɛ-, -oʊ-/ Help:Pronunciation respelling key, ''ih-NAN-tee-ə-mər''), also known ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins. The International Union of Pure and Applied Chemistry (IUPAC) Preferred IUPAC name, recommends using the name "alkene" only for Open-chain compound, acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for Cyclic compound, cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with ''n'' being a >1 natural number (which is two hydrogens less than the corresponding alkane). When ''n'' is four or more, isomers are possible, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Group
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula (whereas normal methane has the formula ). In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many organic compounds. It is a very stable group in most molecules. While the methyl group is usually part of a larger molecule, bonded to the rest of the molecule by a single covalent bond (), it can be found on its own in any of three forms: methanide anion (), methylium cation () or methyl radical (). The anion has eight valence electrons, the radical seven and the cation six. All three forms are highly reactive and rarely observed. Methyl cation, anion, and radical Methyl cation The methylium cation () exists in the gas phase, but is otherwise not encountered. Some compounds are considered to be sources of the cation, and this simplification is used pervasively in organic chemistry. For ex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
-ene
The suffix -ene is used in organic chemistry to form names of organic compounds where the -C=C- group has been attributed the highest priority according to the rules of organic nomenclature. Sometimes a number between hyphens is inserted before it to say that the double bond is between that atom and the atom with the next number up. This suffix comes from the end of the word ethylene, which is the simplest alkene. The final "-e" disappears if it comes before by a suffix that starts with a vowel, e.g. "-enal", which is a compound that contains both a -C=C- bond and an aldehyde functional group. If the other suffix starts with a consonant or "y", the final "-e" remains, ''e.g.'' "-enediyne" (which has the "-ene" suffix and also the " -yne" suffix, for a compound with a double bond and two triple bonds.) A Greek number prefix before the "-ene" indicates how many double bonds there are in the compound, e.g. butadiene. The suffix "-ene" is also used in inorganic chemistry to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IUPAC Nomenclature Of Organic Chemistry
In chemical nomenclature, the IUPAC nomenclature of organic chemistry is a method of naming organic chemical compounds as recommended by the International Union of Pure and Applied Chemistry (IUPAC). It is published in the '' Nomenclature of Organic Chemistry'' (informally called thBlue Book. Ideally, every possible organic compound should have a name from which an unambiguous structural formula can be created. There is also an IUPAC nomenclature of inorganic chemistry. To avoid long and tedious names in normal communication, the official IUPAC naming recommendations are not always followed in practice, except when it is necessary to give an unambiguous and absolute definition to a compound. IUPAC names can sometimes be simpler than older names, as with ethanol, instead of ethyl alcohol. For relatively simple molecules they can be more easily understood than non-systematic names, which must be learnt or looked over. However, the common or trivial name is often substantially ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Structural Isomer
In chemistry, a structural isomer (or constitutional isomer in the IUPAC nomenclature) of a compound is a compound that contains the same number and type of atoms, but with a different connectivity (i.e. arrangement of bonds) between them. The term metamer was formerly used for the same concept. For example, butanol , methyl propyl ether , and diethyl ether have the same molecular formula but are three distinct structural isomers. The concept applies also to polyatomic ions with the same total charge. A classical example is the cyanate ion and the fulminate ion . It is also extended to ionic compounds, so that (for example) ammonium cyanate and urea are considered structural isomers,William F. Bynum, E. Janet Browne, Roy Porter (2014)''Dictionary of the History of Science'' page 218. and so are methylammonium formate and ammonium acetate . Structural isomerism is the most radical type of isomerism. It is opposed to stereoisomerism, in which the atoms and bon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |