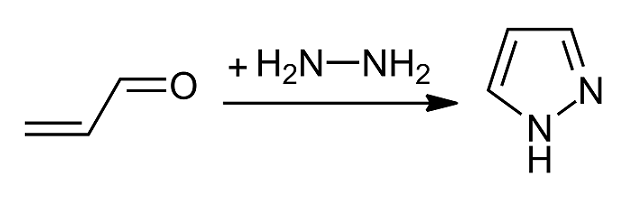|
Methyl Dehydroabietate
Methyl dehydroabietate is a methyl ester derivative of dehydroabietic acid, a naturally occurring resin acid found in coniferous trees. It is characterized by a tricyclic diterpenoid structure and is commonly used in the synthesis of various chemical derivatives. Chemical structure and properties Methyl dehydroabietate has the molecular formula C21H30O2 and a molecular weight of 314.46 g/mol. It appears as a white to off-white solid with a boiling point of 390.2 °C at 760 mmHg and a flash point of 184.3 °C. The compound is practically insoluble in water but soluble in organic solvents such as ethanol and DMSO. Synthesis and derivatives It can be synthesized through the esterification of dehydroabietic acid. Various derivatives have been developed for research purposes. For instance, chalcone and pyrazole Pyrazole is an organic compound with the chemical formula, formula . It is a heterocycle characterized as an azole with a 5-membered ring of three carbon atoms and two adjace ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dehydroabietic Acid
Dehydroabietic acid (DHA) is a naturally occurring abietane-type diterpenoid resin acid Resin acid refers to any of several related carboxylic acids found in tree resins. Nearly all resin acids have the same basic skeleton: three fused rings having the empirical formula C19H29COOH. Resin acids occur in nature as tacky, yellowish gum ... found predominantly in coniferous trees. It is a major component of rosin and is utilized in various industrial applications due to its chemical properties. Chemical structure and properties Dehydroabietic acid has the molecular formula C20H28O2 and a molecular weight of 300.44 g/mol. It appears as a white to off-white solid with a melting point of 150–153 °C and a boiling point of approximately 390 °C. The compound is practically insoluble in water but soluble in organic solvents such as ethanol and acetone. Biological activity It exhibits a range of biological activities, including: * Anti-inflammatory: Acts as a dual activator of peroxis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Resin Acid
Resin acid refers to any of several related carboxylic acids found in tree resins. Nearly all resin acids have the same basic skeleton: three fused rings having the empirical formula C19H29COOH. Resin acids occur in nature as tacky, yellowish gums consisting of several compounds. They are water-insoluble. A common resin acid is abietic acid. Resin acids are used to produce soaps for diverse applications, but their use is being displaced increasingly by synthetic acids such as 2-ethylhexanoic acid or petroleum-derived naphthenic acids. Botanical analysis Resin acids are protectants and wood preservatives that are produced by parenchymatous epithelial cells that surround the resin ducts in trees from temperate coniferous forests. The resin acids are formed when two-carbon and three-carbon molecules couple with isoprene building units to form monoterpenes (volatile), sesquiterpenes (volatile), and diterpenes (nonvolatile) structures. Pines contain numerous vertical and radial r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Esterification
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distinctive functional group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well (e.g. amides), but not according to the IUPAC. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils. Lactones are cyclic carboxylic esters; naturally occurring lactones are mainly 5- and 6-membered ring lactones. Lactones contribute to the aroma of fruits, butter, cheese, vegetables like celery and other foods. Esters can be formed from oxoacids (e.g. esters of acetic acid, carbonic acid, sulfu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chalcone
Chalcone is the organic compound C6H5C(O)CH=CHC6H5. It is an α,β-unsaturated ketone. A variety of important biological compounds are known collectively as chalcones or chalconoids. They are widely known bioactive substances, fluorescent materials, and chemical intermediates. Chemical properties Chalcones have two absorption maxima at 280 nm and 340 nm. Biosynthesis Chalcones and chalconoids are synthesized in plants as secondary metabolites. The enzyme chalcone synthase, a type III polyketide synthase, is responsible for the biosynthesis of these compounds. The enzyme is found in all "higher" (vascular) and several "lower" ( non-vascular) plants. Laboratory synthesis Chalcone is usually prepared by an aldol condensation between benzaldehyde and acetophenone. : This reaction, which can be carried out without any solvent, is so reliable that it is often given as an example of green chemistry in undergraduate education. Potential pharmacology Chalcones and thei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrazole
Pyrazole is an organic compound with the chemical formula, formula . It is a heterocycle characterized as an azole with a 5-membered ring of three carbon atoms and two adjacent nitrogen atoms, which are in Arene substitution pattern, ortho-substitution. Pyrazoles are also a class of compounds that have the ring C3N2 with adjacent nitrogen atoms. Pyrazole itself has few applications but many substituted pyrazoles are of commercial interest. Notable drugs containing a pyrazole ring are celecoxib (celebrex) and the anabolic steroid stanozolol. Properties Pyrazole is a weak base, with p''K''b 11.5 (p''K''a of the conjugate acid 2.49 at 25 °C). According to X-ray crystallography, the compound is planar. The two C-N distances are similar, both near 1.33 Å History The term pyrazole was given to this class of compounds by German Chemist Ludwig Knorr in 1883. In a classical method developed by German chemist Hans von Pechmann in 1898, pyrazole was synthesized from acetylene and di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diterpenes
Diterpenes are a class of terpenes composed of four isoprene units, often with the molecular formula C20H32. They are biosynthesized by plants, animals and fungi via the HMG-CoA reductase pathway, with geranylgeranyl pyrophosphate being a primary intermediate. Diterpenes form the basis for biologically important compounds such as retinol, retinal, and phytol. Some diterpenes are known to be antimicrobial and anti-inflammatory. Structures As with most terpenes a huge number of potential structures exists, which may be broadly divided according to the number of rings present. Biosynthesis Diterpenes are derived from the addition of one IPP unit to FPP to form geranylgeranyl pyrophosphate (GGPP). From GGPP, structural diversity is achieved mainly by two classes of enzymes; the diterpene synthases and cytochromes P450. Several diterpenes are produced by plants and cyanobacteria. GGPP is also the precursor for the synthesis of the phytane by the action of the enzyme geran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isopropyl Compounds
In organic chemistry, a propyl group is a three-carbon alkyl substituent with chemical formula for the linear form. This substituent form is obtained by removing one hydrogen atom attached to the terminal carbon of propane. A propyl substituent is often represented in organic chemistry with the symbol Pr (not to be confused with the element praseodymium). An isomeric form of propyl is obtained by moving the point of attachment from a terminal carbon atom to the central carbon atom, named isopropyl or 1-methylethyl. To maintain four substituents on each carbon atom, one hydrogen atom has to be moved from the middle carbon atom to the carbon atom which served as attachment point in the ''n''-propyl variant, written as . Linear propyl is sometimes termed normal and hence written with a prefix ''n''- (i.e., ''n-''propyl), as the absence of the prefix ''n''- does not indicate which attachment point is chosen, i.e. absence of prefix does not automatically exclude the possibility of i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Esters
In chemistry, an ester is a chemical compound, compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a Functional group#Groups containing oxygen , distinctive functional group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well (e.g. amides), but not according to the IUPAC. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils. Lactones are cyclic carboxylic esters; naturally occurring lactones are mainly 5- and 6-membered ring lactones. Lactones contribute to the aroma of fruits, butter, cheese, vegetables like celery and other foods. Esters can be formed from oxoa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Esters
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom chemical bond, bonded to three hydrogen atoms, having chemical formula (whereas normal methane has the formula ). In chemical formula, formulas, the group is often skeletal formula#Pseudoelement symbols, abbreviated as Me. This hydrocarbon group occurs in many organic compounds. It is a very stable group in most molecules. While the methyl group is usually part of a larger molecule, bonded to the rest of the molecule by a single covalent bond (), it can be found on its own in any of three forms: methanide anion (), methylium cation () or methyl radical (chemistry), radical (). The anion has eight valence electrons, the radical seven and the cation six. All three forms are highly reactive and rarely observed. Methyl cation, anion, and radical Methyl cation The methylium cation () exists in the gas phase, but is otherwise not encountered. Some compounds are considered to be sources ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |