|
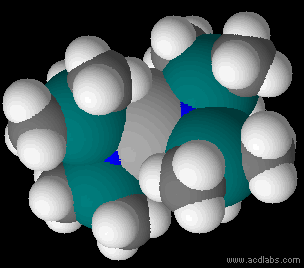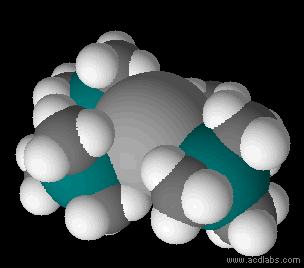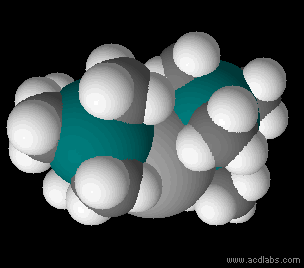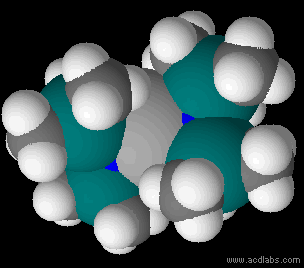
Metal Bis(trimethylsilyl)amides
Metal bis(trimethylsilyl)amides (often abbreviated as metal silylamides) are coordination complexes composed of a cationic metal M with anionic bis(trimethylsilyl)amide ligands (the Valence (chemistry)#monovalent, monovalent anion, or monovalent group, and are part of a broader category of metal amides. Due to the bulky hydrocarbon backbone metal bis(trimethylsilyl)amide complexes have low lattice energies and are lipophilic. For this reason, they are soluble in a range of solvent#Physical properties of common solvents, nonpolar organic solvents, in contrast to simple metal halides, which only dissolve in reactive solvents. These steric bulky complexes are molecular, consisting of mono-, di-, and tetramers. Having a built-in base, these compounds conveniently react with even weakly protic reagents. The class of ligands and pioneering studies on their coordination compounds were described by Bürger and Wannagat. The ligands are often denoted ''hmds'' (e.g. M(N(SiMe3)2)3 = M(hmds) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MN(tms)2
MN may refer to: Places * Mongolia (ISO 3166-1 country code) * Montenegro (former ISO 3166 country code) * Monaco (FIPS 10-4 country code) * Minnesota, US (postal abbreviation) * Manipur, a state in northeast India * Province of Mantua, or of Mantova, in Italy * County Monaghan, in Ireland (license plate code) Language * Mongolian language (ISO 639-1 code) * mn (digraph), ''mn'' (digraph), a combination of letters used in spelling Science and technology * Manganese, symbol Mn, a chemical element * .mn, the Internet country code top-level domain for Mongolia * mega-, MegaNewton (unit), newton (MN), a unit of force equal to one million newtons * milli-, milliNewton (unit) , newton (mN), one-thousandth of a newton * Membranous nephropathy * Minimum mode, a hardware mode available to Intel 8086 and 8088 processors * Number average molecular weight (Mn) * Mammal Neogene zones, a system of biostratigraphic zones in the stratigraphic record used to correlate mammal-bearing fossil localit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organometallics
''Organometallics'' is a biweekly journal published by the American Chemical Society. Its area of focus is organometallic and organometalloid chemistry. This peer-reviewed journal has an impact factor of 3.837 as reported by the 2021 Journal Citation Reports by Thomson Reuters. Since 2015 Paul Chirik is the editor-in-chief of ''Organometallics''. He is an American chemist and the Edwards S. Sanford Professor of Chemistry at Princeton University, and associate director for external partnerships of the Andlinger Center for Energy and the Environment. He writes about the catalysis of hydrocarbons. Past editors-in-chief are Dietmar Seyferth and John Gladysz. Retrieved on 2014-07-30. This journal is indexed in |
Selenium Tetrachloride
Selenium tetrachloride is the inorganic compound composed with the formula SeCl4. This compound exists as yellow to white volatile solid. It is one of two commonly available selenium chlorides, the other example being selenium monochloride, Se2Cl2. SeCl4 is used in the synthesis of other selenium compounds. Synthesis and structure The compound is prepared by treating selenium with chlorine. When the reacting selenium is heated, the product sublimes from the reaction flask. The volatility of selenium tetrachloride can be exploited to purification of selenium. Solid SeCl4 is actually a tetrameric cubane-type cluster, for which the Se atom of an SeCl6 octahedron sits on four corners of the cube and the bridging Cl atoms sit on the other four corners. The bridging Se-Cl distances are longer than the terminal Se-Cl distances, but all Cl-Se-Cl angles are approximately 90°. SeCl4 has often been used as an example for teaching VSEPR rules of hypervalent molecules. As such, o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetraselenium Tetranitride
Tetraselenium tetranitride is the inorganic compound with the formula . Like the analogous tetrasulfur tetranitride , is an orange solid. It is however less soluble and more shock-sensitive than . As determined by X-ray crystallography, adopts a cage structure similar to that of . The Se−Se and Se−N distances are 2.740 and 1.800 Å, respectively. The N−Se−N angles are 90°. Among its many reactions, reacts with aluminium chloride Aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula . It forms a hexahydrate with the formula , containing six water molecules of hydration. Both the anhydrous form and the hexahydrate are col ... to form adducts of . References {{Nitrides Explosive chemicals Inorganic compounds Nitrides Eight-membered rings Nitrogen heterocycles Heterocyclic compounds with 3 rings Selenium compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Syntheses
''Inorganic Syntheses'' is a book series which aims to publish "detailed and foolproof" procedures for the synthesis of inorganic compounds. Although this series of books are edited, they usually are referenced like a journal, without mentioning the names of the checkers (referees) or the editor. A similar format is usually followed for the series '' Organic Syntheses''. Volumes See also * Organic SynthesesReferences {{chem-book-stub Book series introduced in 1939 ...[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trimethylsilyl Chloride
Trimethylsilyl chloride, also known as chlorotrimethylsilane is an organosilicon compound ( silyl halide), with the formula , often abbreviated or TMSCl. It is a colourless volatile liquid that is stable in the absence of water. It is widely used in organic chemistry. Preparation TMSCl is prepared on a large scale by the '' direct process'', the reaction of methyl chloride with a silicon-copper alloy. The principal target of this process is dimethyldichlorosilane, but substantial amounts of the trimethyl and monomethyl products are also obtained. The relevant reactions are (Me = methyl, ): x\ \ce \longrightarrow \begin \ce, \\ pt \ce, \\ pt \ce,\\ pt \text \end Typically about 2–4% of the product stream is the monochloride, which forms an azeotrope with . Reactions and uses TMSCl is reactive toward nucleophiles, resulting in the replacement of the chloride. In a characteristic reaction of TMSCl, the nucleophile is water, resulting in hydrolysis to give the hexa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur Dichloride
Sulfur dichloride is the chemical compound with the formula . This cherry-red liquid is the simplest sulfur chloride and one of the most common, and it is used as a precursor to organosulfur compounds. It is a highly corrosive and toxic substance, and it reacts on contact with water to form chlorine-containing acids. Chlorination of sulfur is produced by the chlorination of either elemental sulfur or disulfur dichloride. The process occurs in a series of steps, some of which are: :; ''ΔH'' = −58.2 kJ/mol :; ''ΔH'' = −40.6 kJ/mol The addition of to has been proposed to proceed via a mixed valence intermediate . undergoes even further chlorination to give , but this species is unstable at near room temperature. It is likely that several exist where ''n'' > 2. Disulfur dichloride, , is a common impurity in . Separation of from is possible via distillation with to form an azeotrope of 99% purity. Sulfur dichloride loses chlorine slowly at room temperature, convert ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrasulfur Tetranitride
Tetrasulfur tetranitride is an inorganic compound with the formula . This vivid orange, opaque, crystalline explosive is the most important binary sulfur nitride, which are compounds that contain only the elements sulfur and nitrogen. It is a precursor to many S-N compounds and has attracted wide interest for its unusual structure and bonding. Nitrogen and sulfur have similar electronegativities. When the properties of atoms are so highly similar, they often form extensive families of covalently bonded structures and compounds. Indeed, a large number of S-N and S-NH compounds are known with as their parent. Structure adopts an unusual "extreme cradle" structure, with D2d point group symmetry. It can be viewed as a derivative of a (hypothetical) eight-membered ring (or more simply a 'deformed' eight-membered ring) of alternating sulfur and nitrogen atoms. The pairs of sulfur atoms across the ring are separated by 2.586 Å, resulting in a cage-like structure as determine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Aluminium Hydride
Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula or . It is a white solid, discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters, carboxylic acids, and amides. The solid is dangerously reactive toward water, releasing gaseous hydrogen (H2). Some related derivatives have been discussed for hydrogen storage. Properties, structure, preparation LAH is a colourless solid but commercial samples are usually gray due to contamination. This material can be purified by recrystallization from diethyl ether. Large-scale purifications employ a Soxhlet extractor. Commonly, the impure gray material is used in synthesis, since the impurities are innocuous and can be easily separated from the organic products. The pure powdered material is pyrophoric, but not its large crystals. Some commercial materials contain mineral oil to inhibit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transmetallation
Transmetalation (alt. spelling: transmetallation) is a type of organometallic reaction that involves the transfer of ligands from one metal to another. It has the general form: :M1–R + M2–R′ → M1–R′ + M2–R where R and R′ can be, but are not limited to, an alkyl, aryl, alkynyl, allyl, halogen, or pseudohalogen group. The reaction is usually an irreversible process due to thermodynamic and kinetic reasons. Thermodynamics will favor the reaction based on the electronegativities of the metals and kinetics will favor the reaction if there are empty orbitals on both metals. There are different types of transmetalation including redox-transmetalation and redox-transmetalation/ligand exchange. During transmetalation the metal-carbon bond is activated, leading to the formation of new metal-carbon bonds. Transmetalation is commonly used in catalysis, synthesis of main group complexes, and synthesis of transition metal complexes. Types of transmetalation There are two main t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethoxyethane
Dimethoxyethane, also known as glyme, monoglyme, dimethyl glycol, ethylene glycol dimethyl ether, dimethyl cellosolve, and DME, is a colorless, aprotic, and liquid ether that is used as a solvent, especially in batteries. Dimethoxyethane is miscible with water. Production Monoglyme is produced industrially by the reaction of dimethylether with ethylene oxide: :CH3OCH3 + CH2CH2O → CH3OCH2CH2OCH3 Applications as solvent and ligand left, 144px, Structure of the coordination complex NbCl3(dimethoxyethane)(3-hexyne). Together with a high-permittivity solvent (e.g. propylene carbonate), dimethoxyethane is used as the low-viscosity component of the solvent for electrolytes of lithium batteries. In the laboratory, DME is used as a coordinating solvent. Dimethoxyethane is often used as a higher-boiling-point alternative to diethyl ether and tetrahydrofuran. Dimethoxyethane acts as a bidentate ligand for some metal cations. It is therefore often used in organometallic chemist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dibutylmagnesium
Dibutylmagnesium is an organometallic chemical compound of magnesium. Its chemical formula is . Dibutylmagnesium is a chemical compound from the group of organomagnesium compounds. The pure substance is a waxy solid. Commercially, it is marketed as solution in heptane. Synthesis Dibutylmagnesium can be obtained by reaction of butyllithium with butylmagnesium chloride and subsequent addition of magnesium 2-ethylhexanoate. The compound can also be prepared by hydrogenation of magnesium, followed by reaction with 1-butene. It is also possible to prepare dibutylmagnesium using 2-chlorobutane, magnesium powder, and ''n''-butyllithium. Use Dibutylmagnesium is used as a convenient reagent for the preparation of organomagnesium compounds. References Terry L. Rathman: "Dibutylmagnesium". In: '' Encyclopedia of Reagents for Organic Synthesis'', 2001, doi:10.1002/047084289X.rd063 Alan W. Duff, Peter B. Hitchcock, ''et al'': "'Dibutylmagnesium', a convenient re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |