|
Membrane Vesicle Trafficking
Membrane vesicle trafficking in eukaryotic animal cells involves movement of biochemical signal molecules from synthesis-and-packaging locations in the Golgi body to specific release locations on the inside of the plasma membrane of the secretory cell. It takes place in the form of Golgi membrane-bound micro-sized vesicles, termed membrane vesicles (MVs). In this process, the packed cellular products are released or secreted outside the cell, across its membrane. On the other hand, the vesicular membrane is retained and recycled by the secretory cells. This phenomenon has a major role in synaptic neurotransmission, endocrine secretion, mucous secretion, granular-product secretion by neutrophils, and other phenomena. The scientists behind this discovery were awarded Nobel Prize for the year 2013. In prokaryotic, gram-negative bacterial cells, membrane vesicle trafficking is mediated through bacterial outer membrane bounded nano-sized vesicles, called outer membrane vesicles (OMVs). ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Eukaryotic
The eukaryotes ( ) constitute the Domain (biology), domain of Eukaryota or Eukarya, organisms whose Cell (biology), cells have a membrane-bound cell nucleus, nucleus. All animals, plants, Fungus, fungi, seaweeds, and many unicellular organisms are eukaryotes. They constitute a major group of Outline of life forms, life forms alongside the two groups of prokaryotes: the Bacteria and the Archaea. Eukaryotes represent a small minority of the number of organisms, but given their generally much larger size, their collective global biomass is much larger than that of prokaryotes. The eukaryotes emerged within the archaeal Kingdom (biology), kingdom Asgard (Archaea), Promethearchaeati and its sole phylum Promethearchaeota. This implies that there are only Two-domain system, two domains of life, Bacteria and Archaea, with eukaryotes incorporated among the Archaea. Eukaryotes first emerged during the Paleoproterozoic, likely as Flagellated cell, flagellated cells. The leading evolutiona ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Exocytosis
Exocytosis is a term for the active transport process that transports large molecules from cell to the extracellular area. Hormones, proteins and neurotransmitters are examples of large molecules that can be transported out of the cell. Exocytosis is a crucial transport mechanism that enables polar molecules to flow through the cell membranes’ hydrophobic lipid bilayer. The transport process is essential to hormone secretion, immune response and neurotransmission. Both prokaryotes and eukaryotes undergo exocytosis. Prokaryotes secrete molecules and cellular waste through translocons that are localized to the cell membrane. In addition, they secrete molecules to other cells through specialized organs. Eukaryotes rely on multiple cellular processes to perform the exocytosis process. Eukaryotes have several organelles and a nucleus in the cytoplasm that are connected through multiple transport routes, that is formally known as the secretory pathway. This is a complex pathway with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microtubule
Microtubules are polymers of tubulin that form part of the cytoskeleton and provide structure and shape to eukaryotic cells. Microtubules can be as long as 50 micrometres, as wide as 23 to 27 nanometer, nm and have an inner diameter between 11 and 15 nm. They are formed by the polymerization of a Protein dimer, dimer of two globular proteins, Tubulin#Eukaryotic, alpha and beta tubulin into #Structure, protofilaments that can then associate laterally to form a hollow tube, the microtubule. The most common form of a microtubule consists of 13 protofilaments in the tubular arrangement. Microtubules play an important role in a number of cellular processes. They are involved in maintaining the structure of the cell and, together with microfilaments and intermediate filaments, they form the cytoskeleton. They also make up the internal structure of cilia and flagella. They provide platforms for intracellular transport and are involved in a variety of cellular processes, in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
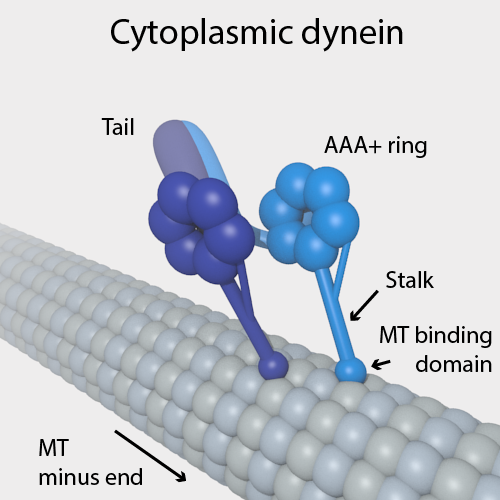
Dynein
Dyneins are a family of cytoskeletal motor proteins (though they are actually protein complexes) that move along microtubules in cells. They convert the chemical energy stored in ATP to mechanical work. Dynein transports various cellular cargos, provides forces and displacements important in mitosis, and drives the beat of eukaryotic cilia and flagella. All of these functions rely on dynein's ability to move towards the minus-end of the microtubules, known as retrograde transport; thus, they are called "minus-end directed motors". In contrast, most kinesin motor proteins move toward the microtubules' plus-end, in what is called anterograde transport. Classification Dyneins can be divided into two groups: cytoplasmic dyneins and axonemal dyneins, which are also called ciliary or flagellar dyneins. * cytoplasmic ** heavy chain: DYNC1H1, DYNC2H1 ** intermediate chain: DYNC1I1, DYNC1I2 ** light intermediate chain: DYNC1LI1, DYNC1LI2, DYNC2LI1 ** light chain: DYNLL1, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kinesin
A kinesin is a protein complex belonging to a class of motor proteins found in eukaryotic cells. Kinesins move along microtubule (MT) filaments and are powered by the hydrolysis of adenosine triphosphate (ATP) (thus kinesins are ATPases, a type of enzyme). The active movement of kinesins supports several cellular functions including mitosis, meiosis and transport of cellular cargo, such as in axonal transport, and intraflagellar transport. Most kinesins walk towards the plus end of a microtubule, which, in most cells, entails transporting cargo such as protein and membrane components from the center of the cell towards the periphery. This form of transport is known as anterograde transport. In contrast, dyneins are motor proteins that move toward the minus end of a microtubule in retrograde transport. Discovery The first kinesins to be discovered were microtubule-based anterograde intracellular transport motors in 1985, based on their motility in cytoplasm extruded from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Myosin
Myosins () are a Protein family, family of motor proteins (though most often protein complexes) best known for their roles in muscle contraction and in a wide range of other motility processes in eukaryotes. They are adenosine triphosphate, ATP-dependent and responsible for actin-based motility. The first myosin (M2) to be discovered was in 1864 by Wilhelm Kühne. Kühne had extracted a viscous protein from skeletal muscle that he held responsible for keeping the tension state in muscle. He called this protein ''myosin''. The term has been extended to include a group of similar ATPases found in the cell (biology), cells of both striated muscle tissue and smooth muscle tissue. Following the discovery in 1973 of enzymes with myosin-like function in ''Acanthamoeba, Acanthamoeba castellanii'', a global range of divergent myosin genes have been discovered throughout the realm of eukaryotes. Although myosin was originally thought to be restricted to muscle cells (hence ''wikt:myo-#Pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
COPII
The coat protein complex II, or COPII, is a group of proteins that facilitate the formation of vesicles to transport proteins from the endoplasmic reticulum to the Golgi apparatus or endoplasmic-reticulum–Golgi intermediate compartment. This process is termed anterograde transport, in contrast to the retrograde transport associated with the COPI complex. COPII is assembled in two parts: first an inner layer of Sar1, Sec23, and Sec24 forms; then the inner coat is surrounded by an outer lattice of Sec13 and Sec31. Function The COPII coat is responsible for the formation of vesicles from the endoplasmic reticulum (ER). These vesicles transport cargo proteins to the Golgi apparatus (in yeast) or the endoplasmic-reticulum-Golgi intermediate compartment (ERGIC, in mammals). Coat assembly is initiated when the cytosolic Ras GTPase Sar1 is activated by its guanine nucleotide exchange factor Sec12. Activated Sar1-GTP inserts itself into the ER membrane, binding preferentially to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
COPI
COPI is a coatomer, a protein complex that coats vesicle (biology), vesicles transporting proteins from the ''cis'' end of the Golgi complex back to the rough endoplasmic reticulum (ER), where they were originally Translation (genetics), synthesized, and between Golgi compartments. This type of transport is ''retrograde transport'', in contrast to the ''anterograde transport'' associated with the COPII protein. The name "COPI" refers to the specific coat protein complex that initiates the budding process on the ''cis''-Golgi membrane. The coat consists of large protein subcomplexes that are made of seven different protein subunits, namely α, β, β', γ, Archain, δ, ε and COPZ1, ζ. Coat proteins Coat protein, or COPI, is an ADP ribosylation factor (ARF)-dependent protein involved in membrane traffic. COPI was first identified in retrograde traffic from the ''cis''-Golgi to the rough endoplasmic reticulum (ER) and is the most extensively studied of ARF-dependent adaptors. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clathrin
Clathrin is a protein that plays a role in the formation of coated vesicles. Clathrin was first isolated by Barbara Pearse in 1976. It forms a triskelion shape composed of three clathrin heavy chains and three light chains. When the triskelia interact they form a polyhedral lattice that surrounds the vesicle. The protein's name refers to this lattice structure, deriving from Latin ''clathri'', meaning lattice. Barbara Pearse named the protein clathrin at the suggestion of Graeme Mitchison, selecting it from three possible options. Coat-proteins, like clathrin, are used to build small vesicles in order to transport molecules within cells. The endocytosis and exocytosis of vesicles allows cells to communicate, to transfer nutrients, to import signaling receptors, to mediate an immune response after sampling the extracellular world, and to clean up the cell debris left by tissue inflammation. The endocytic pathway can be hijacked by viruses and other pathogens in order to gai ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coatomer
The coatomer is a protein complex that coats membrane-bound transport vesicles. Two types of coatomers are known: *COPI (retrograde transport from trans-Golgi network to cis-Golgi network and endoplasmic reticulum) *COPII (anterograde transport from ER to the cis-Golgi) Coatomers are functionally analogous and evolutionarily homologous to clathrin adaptor proteins, also known as adaptins, which regulate endocytosis from the plasma membrane and transport from the trans-Golgi network to lysosomes. Structure The coatomer protein complex is made up of seven nonidentical protein subunits. These seven nonidentical protein subunits are part of two protein subcomplexes. The first subcomplex consists of Ret1(α-COP), Sec27(β’-COP), and Sec 28(ε-COP). The second subcomplex consists of Sec26 (β-COP), Sec21 (γ-COP), Ret2(δ-COP), and Ret3 (ζ-COP). COPI COPI is a coatomer that coats the vesicles transporting proteins from the Golgi complex to the ER. This pathway is ref ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipid Bilayer Fusion
In membrane biology, fusion is the process by which two initially distinct lipid bilayers merge their hydrophobic cores, resulting in one interconnected structure. If this fusion proceeds completely through both leaflets of both bilayers, an aqueous bridge is formed and the internal contents of the two structures can mix. Alternatively, if only one leaflet from each bilayer is involved in the fusion process, the bilayers are said to be hemifused. In hemifusion, the lipid constituents of the outer leaflet of the two bilayers can mix, but the inner leaflets remain distinct. The aqueous contents enclosed by each bilayer also remain separated. Fusion is involved in many cellular processes, particularly in eukaryotes since the eukaryotic cell is extensively sub-divided by lipid bilayer membranes. Exocytosis, fertilization of an egg (biology), egg by sperm and transport of waste products to the lysosome are a few of the many eukaryotic processes that rely on some form of fusion. Fusion is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Motor Protein
Motor proteins are a class of molecular motors that can move along the cytoskeleton of cells. They do this by converting chemical energy into mechanical work by the hydrolysis of ATP. Cellular functions Motor proteins are the driving force behind most active transport of proteins and vesicles in the cytoplasm. Kinesins and cytoplasmic dyneins play essential roles in intracellular transport such as axonal transport and in the formation of the spindle apparatus and the separation of the chromosomes during mitosis and meiosis. Axonemal dynein, found in cilia and flagella, is crucial to cell motility, for example in spermatozoa, and fluid transport, for example in trachea. The muscle protein myosin "motors" the contraction of muscle fibers in animals. Diseases associated with motor protein defects The importance of motor proteins in cells becomes evident when they fail to fulfill their function. For example, kinesin deficiencies have been identified as the cause for Cha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.png)