|
Medical Isotopes
A medical isotope is an isotope used in medicine. The first uses of isotopes in medicine were in radiopharmaceuticals, and this is still the most common use. However more recently, separated stable isotopes have come into use. Radioactive isotopes Radioactive isotopes are used in medicine for both treatment and diagnostic scans. The most common isotope used in diagnostic scans is Technetium-99m, used in approximately 85% of all nuclear medicine diagnostic scans worldwide. It is used for diagnoses involving a large range of body parts and diseases such as cancers and neurological problems. Another well-known radioactive isotope used in medicine is Iodine-131, which is used as a radioactive label for some radiopharmaceutical therapies or the treatment of some types of thyroid cancer. Non-radioactive isotopes Examples of non-radioactive medical isotopes are: * Deuterium in deuterated drugs * Carbon-13 Carbon-13 (13C) is a natural, stable isotope of carbon with a nucleus conta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Technetium-99m
Technetium-99m (99mTc) is a metastable nuclear isomer of technetium-99 (itself an isotope of technetium), symbolized as 99mTc, that is used in tens of millions of medical diagnostic procedures annually, making it the most commonly used Radiopharmacology, medical radioisotope in the world. Technetium-99m is used as a radioactive tracer and can be detected in the body by medical equipment (gamma cameras). It is well suited to the role, because it emits readily detectable gamma rays with a photon energy of 140 kiloelectronvolt, keV (these 8.8 Picometre, pm photons are about the same wavelength as emitted by conventional X-ray diagnostic equipment) and its half-life for gamma emission is 6.0058 hours (meaning 93.7% of it decays to 99Tc in 24 hours). The relatively "short" physical half-life of the isotope and its biological half-life of 1 day (in terms of human activity and metabolism) allows for scanning procedures which collect data rapidly but keep total patient radiatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemical element), but different nucleon numbers (mass numbers) due to different numbers of neutrons in their nuclei. While all isotopes of a given element have similar chemical properties, they have different atomic masses and physical properties. The term isotope is derived from the Greek roots isos (wikt:ἴσος, ἴσος "equal") and topos (wikt:τόπος, τόπος "place"), meaning "the same place"; thus, the meaning behind the name is that different isotopes of a single element occupy the same position on the periodic table. It was coined by Scottish doctor and writer Margaret Todd (doctor), Margaret Todd in a 1913 suggestion to the British chemist Frederick Soddy, who popularized the term. The number of protons within the atomic nuc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
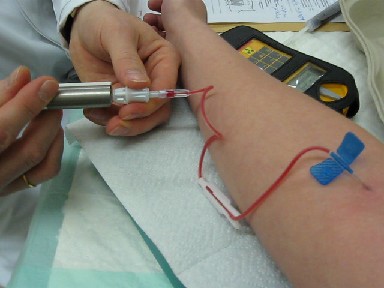
Medicine
Medicine is the science and Praxis (process), practice of caring for patients, managing the Medical diagnosis, diagnosis, prognosis, Preventive medicine, prevention, therapy, treatment, Palliative care, palliation of their injury or disease, and Health promotion, promoting their health. Medicine encompasses a variety of health care practices evolved to maintain and restore health by the prevention (medical), prevention and treatment of illness. Contemporary medicine applies biomedical sciences, biomedical research, medical genetics, genetics, and medical technology to diagnosis (medical), diagnose, treat, and prevent injury and disease, typically through pharmaceuticals or surgery, but also through therapies as diverse as psychotherapy, splint (medicine), external splints and traction, medical devices, biologic medical product, biologics, and Radiation (medicine), ionizing radiation, amongst others. Medicine has been practiced since Prehistoric medicine, prehistoric times, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radiopharmaceutical
Radiopharmaceuticals, or medicinal radiocompounds, are a group of pharmaceutical drugs containing radioactive isotopes. Radiopharmaceuticals can be used as diagnostic and therapeutic agents. Radiopharmaceuticals emit radiation themselves, which is different from contrast media which absorb or alter external electromagnetism or ultrasound. Radiopharmacology is the branch of pharmacology that specializes in these agents. The main group of these compounds are the radiotracers used to diagnose dysfunction in body tissues. While not all medical isotopes are radioactive, radiopharmaceuticals are the oldest and remain the most common of such drugs. Drug nomenclature As with other pharmaceutical drugs, there is standardization of the drug nomenclature for radiopharmaceuticals, although various standards coexist. The International Nonproprietary Names (INNs), United States Pharmacopeia (USP) names, and IUPAC names for these agents are usually similar other than trivial style diffe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotope Separation
Isotope separation is the process of concentrating specific isotopes of a chemical element by removing other isotopes. The use of the nuclides produced is varied. The largest variety is used in research (e.g. in chemistry where atoms of "marker" nuclide are used to figure out reaction mechanisms). By tonnage, separating natural uranium into enriched uranium and depleted uranium is the largest application. In the following text, mainly uranium enrichment is considered. This process is crucial in the manufacture of uranium fuel for nuclear power plants and is also required for the creation of uranium-based nuclear weapons (unless uranium-233 is used). Plutonium-based weapons use plutonium produced in a nuclear reactor, which must be operated in such a way as to produce plutonium already of suitable isotopic mix or ''grade''. While chemical elements can be purified through chemical processes, isotopes of the same element have nearly identical chemical properties which makes this type ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radioactive Decay
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is considered ''radioactive''. Three of the most common types of decay are Alpha decay, alpha, Beta decay, beta, and Gamma ray, gamma decay. The weak force is the Fundamental interactions, mechanism that is responsible for beta decay, while the other two are governed by the electromagnetic force, electromagnetic and nuclear forces. Radioactive decay is a randomness, random process at the level of single atoms. According to quantum mechanics, quantum theory, it is impossible to predict when a particular atom will decay, regardless of how long the atom has existed. However, for a significant number of identical atoms, the overall decay rate can be expressed as a decay constant or as a half-life. The half-lives of radioactive atoms have a huge range: f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodine-131
Iodine-131 (131I, I-131) is an important radioisotope of iodine discovered by Glenn Seaborg and John Livingood in 1938 at the University of California, Berkeley. It has a radioactive decay half-life of about eight days. It is associated with nuclear energy, medical diagnostic and treatment procedures, and natural gas production. It also plays a major role as a radioactive isotope present in nuclear fission products, and was a significant contributor to the health hazards from open-air atomic bomb testing in the 1950s, and from the Chernobyl disaster, as well as being a large fraction of the contamination hazard in the first weeks in the Fukushima nuclear crisis. This is because 131I is a major fission product of uranium and plutonium, comprising nearly 3% of the total products of fission (by weight). See fission product yield for a comparison with other radioactive fission products. 131I is also a major fission product of uranium-233, produced from thorium. Due to its mode of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deuterium
Deuterium (hydrogen-2, symbol H or D, also known as heavy hydrogen) is one of two stable isotopes of hydrogen; the other is protium, or hydrogen-1, H. The deuterium nucleus (deuteron) contains one proton and one neutron, whereas the far more common H has no neutrons. The name ''deuterium'' comes from Greek '' deuteros'', meaning "second". American chemist Harold Urey discovered deuterium in 1931. Urey and others produced samples of heavy water in which the H had been highly concentrated. The discovery of deuterium won Urey a Nobel Prize in 1934. Nearly all deuterium found in nature was synthesized in the Big Bang 13.8 billion years ago, forming the primordial ratio of H to H (~26 deuterium nuclei per 10 hydrogen nuclei). Deuterium is subsequently produced by the slow stellar proton–proton chain, but rapidly destroyed by exothermic fusion reactions. The deuterium–deuterium reaction has the second-lowest energy threshold, and is the most astrophysically acce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deuterated Drug
A deuterated drug is a small molecule medicinal product in which one or more of the hydrogen atoms in the drug molecule have been replaced by its heavier stable isotope deuterium. Because of the kinetic isotope effect, deuterium-containing drugs may have significantly lower rates of metabolism, and hence a longer half-life. Mode of action Hydrogen is a chemical element with an atomic number of 1. It has one proton and one electron. Deuterium is the heavier naturally-occurring stable isotope of hydrogen. Deuterium was discovered by Harold Urey in 1931, for which he received the Nobel Prize in 1934. The deuterium isotope effect has become an important tool in the elucidation of the mechanism of chemical reactions. Deuterium contains one proton, one electron, and a neutron, effectively doubling the mass of the deuterium isotope without changing its properties significantly. However, the C–D bond is a bit shorter, and it has reduced electronic polarizability and less hyperconjugati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon-13
Carbon-13 (13C) is a natural, stable isotope of carbon with a nucleus containing six protons and seven neutrons. As one of the environmental isotopes, it makes up about 1.1% of all natural carbon on Earth. Detection by mass spectrometry A mass spectrum of an organic compound will usually contain a small peak of one mass unit greater than the apparent molecular ion peak (M) of the whole molecule. This is known as the M+1 peak and comes from the few molecules that contain a 13C atom in place of a 12C. A molecule containing one carbon atom will be expected to have an M+1 peak of approximately 1.1% of the size of the M peak, as 1.1% of the molecules will have a 13C rather than a 12C. Similarly, a molecule containing two carbon atoms will be expected to have an M+1 peak of approximately 2.2% of the size of the M peak, as there is double the previous likelihood that any molecule will contain a 13C atom. In the above, the mathematics and chemistry have been simplified, however it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liver Function
Liver function tests (LFTs or LFs), also referred to as a hepatic panel or liver panel, are groups of blood tests that provide information about the state of a patient's liver. These tests include prothrombin time (PT/INR), activated partial thromboplastin time (aPTT), albumin, bilirubin (direct and indirect), and others. The liver transaminases aspartate transaminase (AST or SGOT) and alanine transaminase (ALT or SGPT) are useful biomarkers of liver injury in a patient with some degree of intact liver function. Most liver diseases cause only mild symptoms initially, but these diseases must be detected early. Hepatic (liver) involvement in some diseases can be of crucial importance. This testing is performed on a patient's blood sample. Some tests are associated with functionality (e.g., albumin), some with cellular integrity (e.g., transaminase), and some with conditions linked to the biliary tract ( gamma-glutamyl transferase and alkaline phosphatase). Because some of thes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metabolic Test
''In vitro'' (meaning ''in glass'', or ''in the glass'') studies are performed with cells or biological molecules outside their normal biological context. Colloquially called " test-tube experiments", these studies in biology and its subdisciplines are traditionally done in labware such as test tubes, flasks, Petri dishes, and microtiter plates. Studies conducted using components of an organism that have been isolated from their usual biological surroundings permit a more detailed or more convenient analysis than can be done with whole organisms; however, results obtained from ''in vitro'' experiments may not fully or accurately predict the effects on a whole organism. In contrast to ''in vitro'' experiments, ''in vivo'' studies are those conducted in living organisms, including humans, known as clinical trials, and whole plants. Definition ''In vitro'' (Latin for "in glass"; often not italicized in English usage) studies are conducted using components of an organism that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |