|
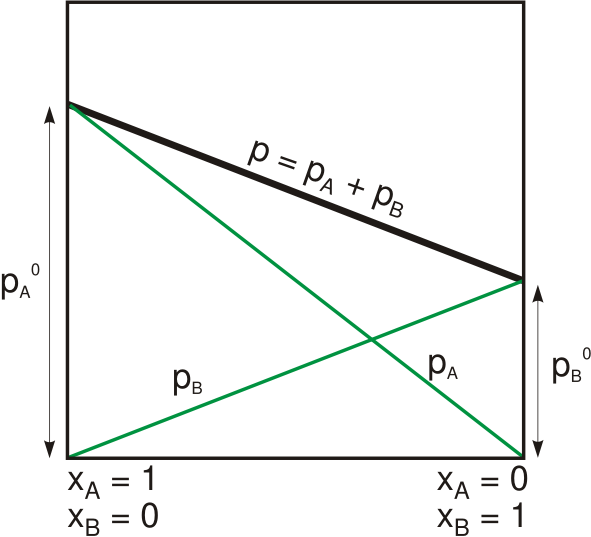
Margules Function
The Margules activity model is a simple thermodynamic model for the excess Gibbs free energy of a liquid mixture introduced in 1895 by Max Margules. After Lewis had introduced the concept of the activity coefficient, the model could be used to derive an expression for the activity coefficients \gamma_i of a compound i in a liquid, a measure for the deviation from ideal solubility, also known as Raoult's law Raoult's law ( law) is a relation of physical chemistry, with implications in thermodynamics. Proposed by French chemist François-Marie Raoult in 1887, it states that the partial pressure of each component of an ideal mixture of ''liquids'' is .... In 1900, Jan Zawidzki proved the model via determining the composition of binary mixtures condensed at different temperatures by their refractive indices. In chemical engineering the Margules Gibbs free energy model for liquid mixtures is better known as the Margules activity or activity coefficient model. Although the model ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gibbs Free Energy
In thermodynamics, the Gibbs free energy (or Gibbs energy as the recommended name; symbol is a thermodynamic potential that can be used to calculate the maximum amount of Work (thermodynamics), work, other than Work (thermodynamics)#Pressure–volume work, pressure–volume work, that may be performed by a closed system, thermodynamically closed system at constant temperature and pressure. It also provides a necessary condition for processes such as chemical reactions that may occur under these conditions. The Gibbs free energy is expressed as G(p,T) = U + pV - TS = H - TS where: * U is the internal energy of the system * H is the enthalpy of the system * S is the entropy of the system * T is the temperature of the system * V is the volume of the system * p is the pressure of the system (which must be equal to that of the surroundings for mechanical equilibrium). The Gibbs free energy change (, measured in joules in International System of Units, SI) is the ''maximum'' amount of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Max Margules
Max Margules (April 23, 1856 – October 4, 1920) was an Austrian mathematician, physicist, and chemist. Margules began his career in research in 1877, when he joined the Central Institute of Meteorology and Geodynamics (ZAMG) in Vienna as a volunteer. After two years, he left Vienna to study in Berlin for a year. He then returned to Vienna and received his PhD in Electrodynamics. During his doctoral studies, he was a Privatdozent ''Privatdozent'' (for men) or ''Privatdozentin'' (for women), abbreviated PD, P.D. or Priv.-Doz., is an academic title conferred at some European universities, especially in German-speaking countries, to someone who holds certain formal qualifi ..., funded entirely by student fees. However, when the administration offered him a teaching job, he refused to convert from Judaism to secure the position, which ended his academic career. In 1882, he returned to ZAMG. During this time he focused on electro- and hydrodynamic problems. In his free time, he ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Activity Coefficient
In thermodynamics, an activity coefficient is a factor used to account for deviation of a mixture of chemical substances from ideal behaviour. In an ideal mixture, the microscopic interactions between each pair of chemical species are the same (or macroscopically equivalent, the enthalpy change of solution and volume variation in mixing is zero) and, as a result, properties of the mixtures can be expressed directly in terms of simple concentrations or partial pressures of the substances present e.g. Raoult's law. Deviations from ideality are accommodated by modifying the concentration by an ''activity coefficient''. Analogously, expressions involving gases can be adjusted for non-ideality by scaling partial pressures by a fugacity coefficient. The concept of activity coefficient is closely linked to that of activity in chemistry. Thermodynamic definition The chemical potential, \mu_\mathrm, of a substance B in an ideal mixture of liquids or an ideal solution is given ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Raoult's Law
Raoult's law ( law) is a relation of physical chemistry, with implications in thermodynamics. Proposed by French chemist François-Marie Raoult in 1887, it states that the partial pressure of each component of an ideal mixture of ''liquids'' is equal to the vapor pressure of the pure component (liquid or solid) multiplied by its mole fraction in the mixture. In consequence, the relative lowering of vapor pressure of a dilute solution of nonvolatile solute is equal to the mole fraction of solute in the solution. Mathematically, Raoult's law for a single component in an ideal solution is stated as : p_i = p_i^\star x_i where p_i is the partial pressure of the component i in the gaseous mixture above the solution, p_i^\star is the equilibrium vapor pressure of the pure component i, and x_i is the mole fraction of the component i in the liquid or solid solution. Where two volatile liquids A and B are mixed with each other to form a solution, the vapor phase consists of both compo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jan Zawidzki
Jan Wiktor Tomasz Zawidzki (December 20, 1866 in Włóki, Masovian Voivodeship – September 14, 1928 in Warsaw) was a Polish physical chemist and historian of chemistry. He researched mainly chemical kinetics, thermochemistry and autocatalysis. Zawidzki was a professor of the Akademia Rolnicza in Dublany (1907–1916), Jagiellonian University (1916–1917), University of Warsaw (1917–1928), rector of the University of Warsaw (1918–1919), member of the Academy of Learning (since 1918), co-founder of the Polish Chemical Society The Polish Chemical Society () is a professional learned society of Polish chemists founded in 1919 to represent the interests of Polish chemists on the local, national and international levels. History The society was founded of 118 Charter Me ... and magazine ''Roczniki Chemii''. Bibliography * ''Kinetyka chemiczna'' (1931) * ''Chemia nieorganiczna'' vol. 1–2 (1932–1936) References * * 1866 births 1928 deaths Historians of science ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
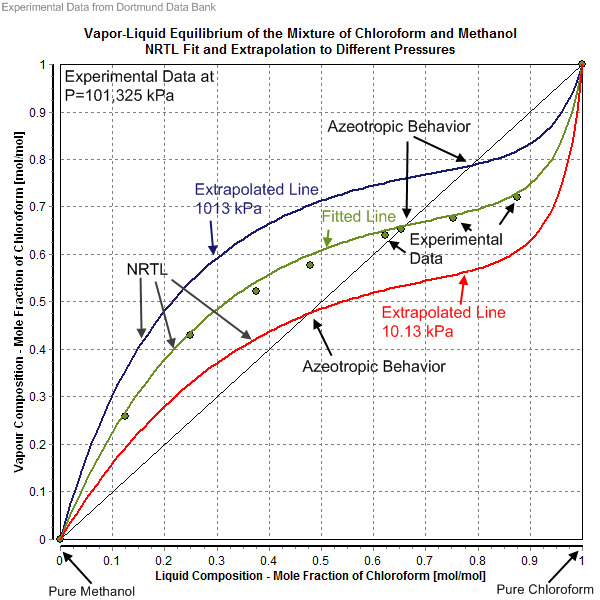
Non-Random Two Liquid Model
The non-random two-liquid model (abbreviated NRTL model) is an activity coefficient model introduced by Renon and Prausnitz in 1968 that correlates the activity coefficients \gamma_i of a compound with its mole fractions x_i in the liquid phase concerned. It is frequently applied in the field of chemical engineering to calculate phase equilibria. The concept of NRTL is based on the hypothesis of Wilson, who stated that the local concentration around a molecule in most mixtures is different from the bulk concentration. This difference is due to a difference between the interaction energy of the central molecule with the molecules of its own kind U_ and that with the molecules of the other kind U_. The energy difference also introduces a non-randomness at the local molecular level. The NRTL model belongs to the so-called local-composition models. Other models of this type are the Wilson model, the UNIQUAC model, and the group contribution model UNIFAC. These local-composition models ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Grant M
Grant or Grants may refer to: People * Grant (given name), including a list of people and fictional characters * Grant (surname), including a list of people and fictional characters ** Ulysses S. Grant (1822–1885), the 18th president of the United States and general of the Union during the American Civil War ** Cary Grant (1904–1986), British-American actor ** Hugh Grant (born 1960), British actor ** Richard E. Grant (born 1957), British-Swazi actor ** Justice Grant (other), judges named Grant * Clan Grant, a Highland Scottish clan Law and philanthropy *Grant (money), an award usually funded by a government, business, or foundation, often with not-for-profit preconditions *Grant (law), a term in conveyancing * Spanish and Mexican land grants in New Mexico * Spanish land grants in Florida *''Grant v Torstar Corp'', a leading Supreme Court of Canada case on responsible communication in the public interest as a defence against defamation Places *Grant County (disamb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gibbs–Duhem Equation
In thermodynamics, the Gibbs–Duhem equation describes the relationship between changes in chemical potential for components in a thermodynamic system: \sum_^I N_i \mathrm\mu_i = - S \mathrmT + V \mathrmp where N_i is the number of moles of component i, \mathrm\mu_i the infinitesimal increase in chemical potential for this component, S the entropy, T the absolute temperature, V volume and p the pressure. I is the number of different components in the system. This equation shows that in thermodynamics intensive properties are not independent but related, making it a mathematical statement of the state postulate. When pressure and temperature are variable, only I-1 of I components have independent values for chemical potential and Gibbs' phase rule follows. The Gibbs−Duhem equation applies to homogeneous thermodynamic systems. It does not apply to inhomogeneous systems such as small thermodynamic systems, systems subject to long-range forces like electricity and gravity, or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chem Eng Sci
''Chemical Engineering Science'' is a peer-reviewed scientific journal covering all aspects of chemical engineering. It is published by Elsevier and was established in 1951. While the journal aims to publish "outstanding research that has as its foundations the Science of Chemical Engineering", it has focuses on the themes of catalysis, green and sustainable science, and environmental and novel materials. The journal publishes full-length research papers, short communications, review articles, and letters to the editors. It offers the choice to publish under both the open access and subscription modes, and follows a single anonymized review process. A related journal, ''Chemical Engineering Science: X'', was discontinued in 2023. Abstracting and indexing The journal is abstracted and indexed in: According to the ''Journal Citation Reports'', the journal has a 2019 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a type of journal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Van Laar Equation
A van is a type of road vehicle used for transporting goods or people. There is some variation in the scope of the word across the different English-speaking countries. The smallest vans, microvans, are used for transporting either goods or people in tiny quantities. Mini MPVs, compact MPVs, and MPVs are all small vans usually used for transporting people in small quantities. Larger vans with passenger seats are used for institutional purposes, such as transporting students. Larger vans with only front seats are often used for business purposes, to carry goods and equipment. Specially equipped vans are used by television stations as mobile studios. Postal services and courier companies use large step vans to deliver packages. Word origin and usage Van meaning a type of vehicle arose as a contraction of the word caravan. The earliest records of a van as a vehicle in English are in the mid-19th century, meaning a covered wagon for transporting goods; the earliest reported rec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Physical Chemistry
Physical chemistry is the study of macroscopic and microscopic phenomena in chemical systems in terms of the principles, practices, and concepts of physics such as motion, energy, force, time, thermodynamics, quantum chemistry, statistical mechanics, analytical dynamics and chemical equilibria. Physical chemistry, in contrast to chemical physics, is predominantly (but not always) a supra-molecular science, as the majority of the principles on which it was founded relate to the bulk rather than the molecular or atomic structure alone (for example, chemical equilibrium and colloids). Some of the relationships that physical chemistry strives to understand include the effects of: # Intermolecular forces that act upon the physical properties of materials ( plasticity, tensile strength, surface tension in liquids). # Reaction kinetics on the rate of a reaction. # The identity of ions and the electrical conductivity of materials. # Surface science and electrochemistry of cell m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |