|
Maraviroc
Maraviroc, sold under the brand names Selzentry (US) and Celsentri (EU), is an antiretroviral medication used to treat HIV infection. It is taken by mouth. It is in the CCR5 receptor antagonist class. It was approved for medical use in the United States in August 2007, and in the European Union in September 2007. Medical uses Maraviroc is indicated, in combination with other antiretroviral medications, for the treatment of only CCR5-tropic HIV-1 infection. Side effects Maraviroc can cause serious, life-threatening side effects. These include liver problems, skin reactions, and allergic reactions. An allergic reaction may happen before liver problems occur. Official labeling of Selzentry has black box warning for hepatotoxicity. The MOTIVATE trials showed no clinically relevant differences in safety between the maraviroc and placebo groups. Mechanism of action Maraviroc is an entry inhibitor. Specifically, maraviroc is a negative allosteric modulator of the CCR5 receptor, w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Discovery And Development Of CCR5 Receptor Antagonists
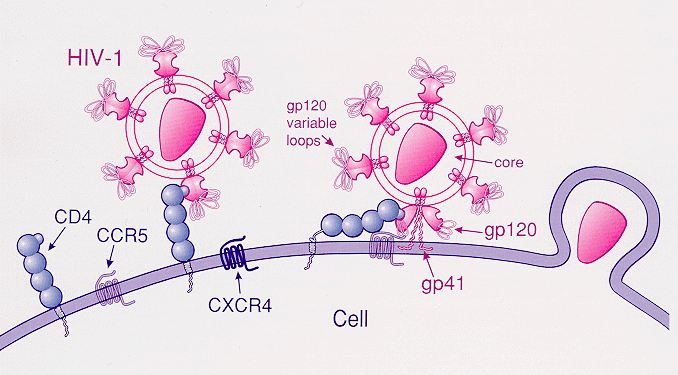
CCR5 receptor antagonists are a class of small molecules that receptor antagonist, antagonize the CCR5 receptor. The C-C motif chemokine receptor CCR5 is involved in the process by which HIV, the virus that causes AIDS, enters cells. Hence antagonists of this receptor are entry inhibitors and have potential therapeutic applications in the treatment of HIV infections. The life cycle of the HIV presents potential targets for drug therapy, one of them being the viral entry pathway. CCR5 and CXCR4 are the main receptors involved in the HIV entry process. These receptors belong to the seven transmembrane G-protein-coupled receptor (GPCR) family and are predominantly expressed on human T-cells, dendritic cells and macrophages, Langerhans cells. They play an important role as co-receptors that HIV type 1 (HIV-1) uses to attach to cells before viral fusion and entry into host cells. HIV isolates can be divided into R5 and X4 strains. R5 strain is when the virus uses the co-receptor CCR5 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Entry Inhibitor
Entry inhibitors, also known as fusion inhibitors, are a class of antiviral drug Antiviral drugs are a class of medication used for treating viral infections. Most antivirals target specific viruses, while a broad-spectrum antiviral is effective against a wide range of viruses. Unlike most antibiotics, antiviral drugs do n ...s that prevent a virus from entering a cell, for example, by blocking a receptor. Entry inhibitors are used to treat conditions such as HIV and hepatitis D. HIV entry They are used in combination therapy for the treatment of HIV infection. This class of drugs interferes with the HIV#Entry to the cell, binding, fusion and entry of an HIV virion to a human cell. By blocking this step in HIV#Replication cycle, HIV's replication cycle, such agents slow the progression from HIV infection to AIDS. Proteins There are several key proteins involved in the HIV#Entry to the cell, HIV entry process. * CD4, a protein receptor found on the surface of helper T c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CCR5
C-C chemokine receptor type 5, also known as CCR5 or CD195, is a protein on the surface of white blood cells that is involved in the immune system as it acts as a receptor for chemokines. In humans, the ''CCR5'' gene that encodes the CCR5 protein is located on the short (p) arm at position 21 on chromosome 3. Certain populations have inherited the ''Delta 32'' mutation, resulting in the genetic deletion of a portion of the CCR5 gene. Homozygous carriers of this mutation are resistant to M-tropic strains of HIV-1 infection. Function The CCR5 protein belongs to the beta chemokine receptors family of integral membrane proteins. It is a G protein–coupled receptor which functions as a chemokine receptor in the CC chemokine group. CCR5's cognate ligands include CCL3, CCL4 (also known as MIP 1''α'' and 1''β'', respectively), and CCL3L1. CCR5 furthermore interacts with CCL5 (a chemotactic cytokine protein also known as RANTES). CCR5 is predominantly expressed on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allosteric Regulation
In biochemistry, allosteric regulation (or allosteric control) is the regulation of an enzyme by binding an effector molecule at a site other than the enzyme's active site. The site to which the effector binds is termed the ''allosteric site'' or ''regulatory site''. Allosteric sites allow effectors to bind to the protein, often resulting in a conformational change and/or a change in protein dynamics. Effectors that enhance the protein's activity are referred to as ''allosteric activators'', whereas those that decrease the protein's activity are called ''allosteric inhibitors''. Allosteric regulations are a natural example of control loops, such as feedback from downstream products or feedforward from upstream substrates. Long-range allostery is especially important in cell signaling. Allosteric regulation is also particularly important in the cell's ability to adjust enzyme activity. The term ''allostery'' comes from the Ancient Greek ''allos'' (), "other", and ''stereos' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Food And Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a federal agency of the Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, caffeine products, dietary supplements, prescription and over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood transfusions, medical devices, electromagnetic radiation emitting devices (ERED), cosmetics, animal foods & feed and veterinary products. The FDA's primary focus is enforcement of the Federal Food, Drug, and Cosmetic Act (FD&C), but the agency also enforces other laws, notably Section 361 of the Public Health Service Act, as well as associated regulations. Much of this regulatory-enforcement work is not directly related to food or drugs, but involves such things as regulating lasers, cellular phones, and condoms, as well as control of disease in contexts v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trofile Assay
HIV tropism refers to the cell type in which the human immunodeficiency virus (HIV) infects and replicates. HIV tropism of a patient's virus is measured by the Trofile assay. HIV can infect a variety of cells such as CD4+ helper T-cells and macrophages that express the CD4 molecule on their surface. HIV-1 entry to macrophages and T helper cells is mediated not only through interaction of the virion envelope glycoproteins (gp120) with the CD4 molecule on the target cells but also with its chemokine coreceptors. Macrophage (M-tropic) strains of HIV-1, or non-syncitia-inducing strains (NSI) use the beta-chemokine receptor CCR5 for entry and are thus able to replicate in macrophages and CD4+ T-cells. These strains are now called R5 viruses. The normal ligands for this receptor—RANTES, macrophage inflammatory protein (MIP)-1β and MIP-1α—are able to suppress HIV-1 infection ''in vitro''. This CCR5 coreceptor is used by almost all primary HIV-1 isolates regardless of viral gen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pfizer
Pfizer Inc. ( ) is an American multinational pharmaceutical and biotechnology corporation headquartered on 42nd Street in Manhattan, New York City. The company was established in 1849 in New York by two German entrepreneurs, Charles Pfizer (1824–1906) and his cousin Charles F. Erhart (1821–1891). Pfizer develops and produces medicines and vaccines for immunology, oncology, cardiology, endocrinology, and neurology. The company has several blockbuster drugs or products that each generate more than billion in annual revenues. In 2020, 52% of the company's revenues came from the United States, 6% came from each of China and Japan, and 36% came from other countries. Pfizer was a component of the Dow Jones Industrial Average stock market index from 2004 to August 2020. The company ranks 64th on the Fortune 500 and 49th on the Forbes Global 2000. History 1849–1950: Early history Pfizer was founded in 1849 by Charles Pfizer and Charles F. Erhart, two cousins w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sandwich, Kent
Sandwich is a town and civil parish in the Dover District of Kent, south-east England. It lies on the River Stour and has a population of 4,985. Sandwich was one of the Cinque Ports and still has many original medieval buildings, including several listed public houses and gates in the old town walls, churches, almshouses and the White Mill. While once a major port, it is now two miles from the sea due to the disappearance of the Wantsum Channel. Its historic centre has been preserved. Sandwich Bay is home to nature reserves and two world-class golf courses, Royal St George's and Prince's. The town is also home to many educational and cultural events. Sandwich also gave its name to the food by way of John Montagu, 4th Earl of Sandwich, and the word ''sandwich'' is now found in several languages. Etymology The place-name 'Sandwich' is first attested in the ''Anglo-Saxon Chronicle'', where it appears as ' in 851 and ' in 993. In the ''Domesday Book'' of 1086 it appears as '. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
European Medicines Agency
The European Medicines Agency (EMA) is an agency of the European Union (EU) in charge of the evaluation and supervision of medicinal products. Prior to 2004, it was known as the European Agency for the Evaluation of Medicinal Products or European Medicines Evaluation Agency (EMEA).Set up by EC Regulation No. 2309/93 as the European Agency for the Evaluation of Medicinal Products, and renamed by EC Regulation No. 726/2004 to the European Medicines Agency, it had the acronym EMEA until December 2009. The European Medicines Agency does not call itself EMA either – it has no official acronym but may reconsider if EMA becomes commonly accepted (secommunication on new visual identity an). The EMA was set up in 1995, with funding from the European Union and the pharmaceutical industry, as well as indirect subsidy from member states, its stated intention to harmonise (but not replace) the work of existing national medicine regulatory bodies. The hope was that this plan would not o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CXCR4
C-X-C chemokine receptor type 4 (CXCR-4) also known as fusin or CD184 (cluster of differentiation 184) is a protein that in humans is encoded by the ''CXCR4'' gene. The protein is a CXC chemokine receptor. Function CXCR-4 is an alpha-chemokine receptor specific for stromal-derived-factor-1 ( SDF-1 also called CXCL12), a molecule endowed with potent chemotactic activity for lymphocytes. CXCR4 is one of several chemokine co-receptors that HIV can use to infect CD4+ T cells. HIV isolates that use CXCR4 are traditionally known as T-cell tropic isolates. Typically, these viruses are found late in infection. It is unclear as to whether the emergence of CXCR4-using HIV is a consequence or a cause of immunodeficiency. CXCR4 is upregulated during the implantation window in natural and hormone replacement therapy cycles in the endometrium, producing, in presence of a human blastocyst, a surface polarization of the CXCR4 receptors suggesting that this receptor is implicated in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Trial
Clinical trials are prospective biomedical or behavioral research studies on human subject research, human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments (such as novel vaccines, pharmaceutical drug, drugs, medical nutrition therapy, dietary choices, dietary supplements, and medical devices) and known interventions that warrant further study and comparison. Clinical trials generate data on dosage, safety and efficacy. They are conducted only after they have received institutional review board, health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted. Depending on product type and development stage, investigators initially enroll volunteers or patients into small Pilot experiment, pi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


.jpg)
.jpg)

_-_geograph.org.uk_-_650895.jpg)
.jpg)