|
Manhattan Project Feed Materials Program
The Manhattan Project feed materials program located and procured uranium ores, and refined and processed them into feed materials for use in the Manhattan Project's isotope enrichment plants at the Clinton Engineer Works in Oak Ridge, Tennessee, and its nuclear reactors at the Hanford Engineer Works in Washington state. The original goal of the feed materials program in 1942 was to acquire approximately of triuranium octoxide () (black oxide). By the time of the dissolution of the Manhattan District on 1 January 1947, it had acquired about , of which came from the Belgian Congo, from the Colorado Plateau, and from Canada. An additional came from "miscellaneous sources", which included quantities recovered from Europe by the Manhattan Project's Alsos Mission. Ores from the Belgian Congo contained the most uranium per mass of rock by far. Much of the mined ore from the Shinkolobwe mine had a black oxide content as high as 65% to 75%, which was many times higher than any ot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranium Processing
Uranium is a chemical element; it has symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium radioactively decays, usually by emitting an alpha particle. The half-life of this decay varies between 159,200 and 4.5 billion years for different isotopes, making them useful for dating the age of the Earth. The most common isotopes in natural uranium are uranium-238 (which has 146 neutrons and accounts for over 99% of uranium on Earth) and uranium-235 (which has 143 neutrons). Uranium has the highest atomic weight of the primordially occurring elements. Its density is about 70% higher than that of lead and slightly lower than that of gold or tungsten. It occurs naturally in low concentrations of a few parts per million in soil, rock and water, and is commercially extracted from uranium-bearing minerals such as uraninite. Many contemporary u ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Major General (United States)
In the United States Armed Forces, a major general is a two-star rank, two-star general officer in the United States United States Army, Army, United States Marine Corps, Marine Corps, United States Air Force, Air Force, and United States Space Force, Space Force. A major general ranks above a Brigadier general (United States), brigadier general and below a Lieutenant general (United States), lieutenant general. The U.S. uniformed services pay grades, pay grade of major general is O-8. It is equivalent to the rank of Rear admiral (United States)#Rear admiral, rear admiral in the other United States Uniformed services of the United States, uniformed services which use Naval officer ranks, naval ranks. It is abbreviated as MG in the Army, MajGen in the Marine Corps, and in the Air Force and Space Force. Major general is the highest permanent peacetime rank that can be conferred upon a commissioned officer in the uniformed services (except when General of the Army (United States ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranium Tetrachloride
Uranium tetrachloride is an inorganic compound, a salt of uranium and chlorine, with the formula UCl4. It is a hygroscopic olive-green solid. It was used in the electromagnetic isotope separation (EMIS) process of uranium enrichment. It is one of the main starting materials for organouranium chemistry. Synthesis and structure Uranium tetrachloride is synthesised generally by the reaction of uranium trioxide (UO3) and hexachloropropene. Solvent UCl4 adducts can be formed by a simpler reaction of UI4 with hydrogen chloride in organic solvents. Uranium tetrachloride also forms the nonahydrate, which can be produced by evaporating a mildly acidic solution of UCl4. According to X-ray crystallography the uranium centers are eight-coordinate, being surrounded by eight chlorine atoms, four at 264 pm and the other four at 287pm. Chemical properties Dissolution in protic solvents is more complicated. When UCl4 is added to water the uranium aqua ion is formed. :UCl4 + H2O → ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ames Process
The Ames process is a process by which pure uranium metal is obtained. It can be achieved by mixing any of the uranium halides (commonly uranium tetrafluoride) with magnesium metal powder or aluminium metal powder. History The Ames process was used on August 3, 1942, by a group of chemists led by Frank Spedding and Harley Wilhelm at the Ames Laboratory as part of the Manhattan Project. It is a type of thermite-based purification, which was patented in 1895 by German chemist Hans Goldschmidt. Development of the Ames process came at a time of increased research into mass uranium-metal production. The desire for increased production was motivated by a fear of Nazi Germany's developing nuclear weapons before the Allies. The process originally involved mixing powdered uranium tetrafluoride and powdered magnesium together. This mixture was placed inside an iron pipe that was welded shut on one side and capped shut on another side. This container, called a "bomb" by Spedding, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ames Project
The Ames Project was a research and development project that was part of the larger Manhattan Project to build the first atomic bombs during World War II. It was founded by Frank Spedding from Iowa State College in Ames, Iowa as an offshoot of the Metallurgical Laboratory at the University of Chicago devoted to chemistry and metallurgy, but became a separate project in its own right. The Ames Project developed the Ames Process, a method for preparing pure uranium metal that the Manhattan Project needed for its atomic bombs and nuclear reactors. Between 1942 and 1945, it produced over of uranium metal. It also developed methods of preparing and casting thorium, cerium and beryllium. In October 1945 Iowa State College received the Army-Navy "E" Award for Excellence in Production, an award usually only given to industrial organizations. In 1947 it became the Ames Laboratory, a national laboratory under the Atomic Energy Commission. Background The discovery of the neutron b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Frank Spedding
Frank Harold Spedding (22 October 1902 – 15 December 1984) was a Canadian-American chemist. He was a renowned expert on rare earth elements, and on extraction of metals from minerals. The uranium extraction process helped make it possible for the Manhattan Project to build the first atomic bombs. A graduate of the University of Michigan and University of California, Berkeley, Spedding became an assistant professor and head of the department of physical chemistry at Iowa State College in 1937. His efforts at building up the school were so successful that he would spend the rest of his career there, becoming a professor of chemistry in 1941, a professor of physics in 1950, a professor of metallurgy in 1962, and ultimately professor emeritus in 1973. He co-founded, along with Dr. Harley Wilhelm, the Institute for Atomic Research and the Ames Laboratory of the Atomic Energy Commission, and directed the Ames Laboratory from its founding in 1947 until 1968. Spedding developed a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
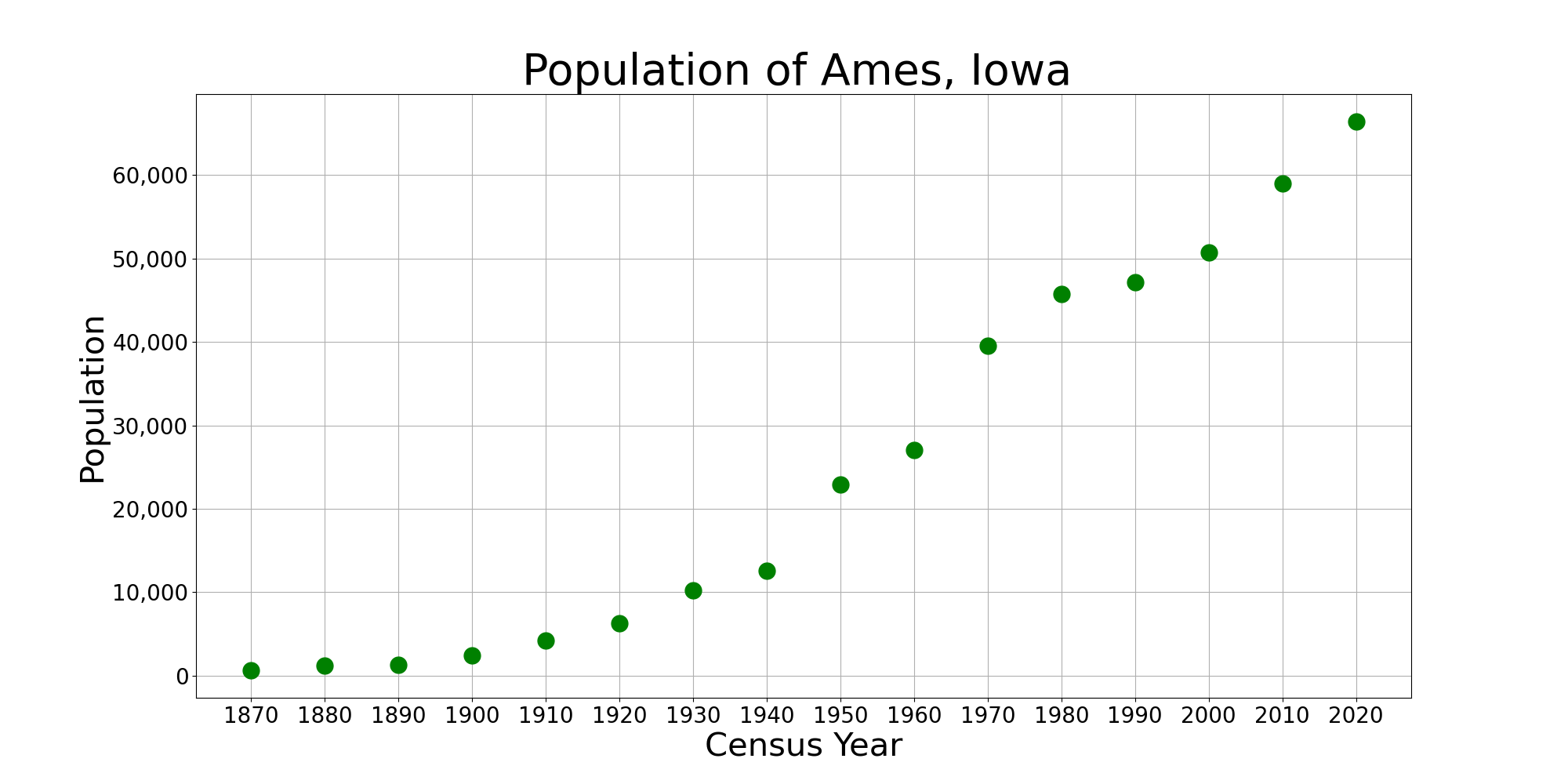
Ames, Iowa
Ames () is a city in Story County, Iowa, United States, located approximately north of Des Moines, Iowa, Des Moines in central Iowa. It is the home of Iowa State University (ISU). According to the 2020 United States census, 2020 census, Ames had a population of 66,427, making it the state's List of cities in Iowa, ninth-most populous city. Iowa State University was home to 30,177 students as of fall 2023, which make up approximately one half of the city's population. A United States Department of Energy national laboratory, Ames Laboratory, is located on the ISU campus. Ames also hosts United States Department of Agriculture (USDA) sites: the largest federal animal disease center in the United States, the USDA Agricultural Research Service's National Animal Disease Center (NADC), as well as one of two national USDA sites for the Animal and Plant Health Inspection Service (APHIS), which comprises the National Veterinary Services Laboratory and the Center for Veterinary Biologics. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iowa State College
Iowa State University of Science and Technology (Iowa State University, Iowa State, or ISU) is a public land-grant research university in Ames, Iowa, United States. Founded in 1858 as the Iowa Agricultural College and Model Farm, Iowa State became one of the nation's first designated land-grant institutions when the Iowa Legislature accepted the provisions of the 1862 Morrill Act on September 11, 1862. On July 4, 1959, the college was officially renamed Iowa State University of Science and Technology. Iowa State is the second largest university in Iowa by total enrollment. The university's academic offerings are administered through eight colleges, including the College of Agriculture and Life Sciences, the College of Veterinary Medicine, the College of Engineering, the Graduate College, the College of Liberal Arts & Sciences, the College of Design, Debbie and Jerry Ivy College of Business, and the College of Health and Human Sciences. They offer more than 100 bachelor' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metallurgical Laboratory
The Metallurgical Laboratory (or Met Lab) was a scientific laboratory from 1942 to 1946 at the University of Chicago. It was established in February 1942 and became the Argonne National Laboratory in July 1946. The laboratory was established in February 1942 to study and use the newly discovered chemical element plutonium. It researched plutonium's chemistry and metallurgy, designed the world's first nuclear reactors to produce it, and developed chemical processes to separate it from other elements. In August 1942 the lab's chemical section was the first to chemically separate a weighable sample of plutonium, and on 2 December 1942, the Met Lab produced the first controlled nuclear chain reaction, in the reactor Chicago Pile-1, which was constructed under the stands of the university's old football stadium, Stagg Field. The Metallurgical Laboratory was established as part of the Metallurgical Project, under the S-1 Committee, and also known as the "Pile" or "X-10" Project, hea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mallinckrodt
Mallinckrodt Pharmaceuticals plc is an American-Irish domiciled manufacturer of specialty pharmaceuticals (namely, adrenocorticotropic hormone), generic drugs and imaging agents. In 2017, it generated 90% of its sales from the U.S. healthcare system. While Mallinckrodt is headquartered in Ireland for tax purposes, its operational headquarters are in the U.S. Mallinckrodt's 2013 tax inversion to Ireland drew controversy when it was shown Acthar was Medicaid's most expensive drug. Mallinckrodt acquires (for repricing), manufactures, and distributes products used in diagnostic procedures and in the treatment of pain and related conditions. This includes the acquisition, manufacture, and distribution of specialty pharmaceuticals, active pharmaceutical ingredients, contrast products, and radiopharmaceuticals. The company employed 5,500 and had net sales of $3.2 billion in 2017, of which $2.9 billion was from the U.S. healthcare system. The company has been implicated as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranium Dioxide
Uranium dioxide or uranium(IV) oxide (), also known as urania or uranous oxide, is an oxide of uranium, and is a black, radioactive, crystalline powder that naturally occurs in the mineral uraninite. It is used in nuclear fuel rods in nuclear reactors. A mixture of uranium and plutonium dioxides is used as MOX fuel. It has been used as an orange, yellow, green, and black color in ceramic glazes and glass. Production Uranium dioxide is produced by reducing uranium trioxide with hydrogen. This reaction often creates triuranium octoxide as an intermediate. :UO3 + H2 → UO2 + H2O at 700 °C (973 K) This reaction plays an important part in the creation of nuclear fuel through nuclear reprocessing and uranium enrichment. Chemistry Structure The solid is isostructural with (has the same structure as) fluorite ( calcium fluoride), where each U is surrounded by eight O nearest neighbors in a cubic arrangement. In addition, the dioxides of cerium, thorium, and the tran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranyl Nitrate
Uranyl nitrate is a water-soluble yellow uranium salt with the formula . The hexa-, tri-, and dihydrates are known. The compound is mainly of interest because it is an intermediate in the preparation of nuclear fuels. In the nuclear industry, it is commonly referred to as yellow salt. Uranyl nitrate can be prepared by reaction of uranium salts with nitric acid. It is soluble in water, ethanol, and acetone. As determined by neutron diffraction, the uranyl center is characteristically linear with short U=O distances. In the equatorial plane of the complex are six U-O bonds to bidentate nitrate and two water ligands. At 245 pm, these U-O bonds are much longer than the U=O bonds of the uranyl center. Uses Processing of nuclear fuels Uranyl nitrate is important for nuclear reprocessing. It is the compound of uranium that results from dissolving the decladded spent nuclear fuel rods or yellowcake in nitric acid, for further separation and preparation of uranium hexafluoride for i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |