|
Manganese Pentacarbonyl Bromide
Manganese pentacarbonyl bromide is an organomanganese compound with the formula BrMn(CO)5. It is a bright orange solid that is a precursor to other manganese complexes. The compound is prepared by treatment of dimanganese decacarbonyl with bromine Bromine is a chemical element; it has chemical symbol, symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between th ...: :Mn2(CO)10 + Br2 → 2 BrMn(CO)5 The complex undergoes substitution by a variety of donor ligands (L), e.g. to give derivatives of the type BrMn(CO)3L2. The complex adopts an octahedral coordination geometry. Manganese pentacarbonyl bromide is a precursor to the arene complexes η6-arene)Mn(CO)3sup>+.S. B. Kim, S. Lotz, S. Sun, Y. K. Chung, R. D. Pike, D. A. Sweigart "Manganese Tricarbonyl Transfer (MTT) Agents" Inorganic Syntheses, 2010, Vol. 35, 109–128. References { ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organomanganese Compound
Organomanganese chemistry is the chemistry Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules a ... of organometallic compounds containing a carbon to manganese chemical bond. In a 2009 review, Cahiez et al. argued that as manganese is cheap and benign (only iron performs better in these aspects), organomanganese compounds have potential as chemical reagents, although currently they are not widely used as such despite extensive research. Synthesis Organomanganese compounds were first reported in 1937 by Gilman and Bailee who described the reaction of phenyllithium and manganese(II) iodide to form phenylmanganese iodide (PhMnI) and diphenylmanganese (Ph2Mn). Following this precedent, other organomanganese halides can be obtained by alkylation of manganese(II) chloride, manganese(II) bromide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimanganese Decacarbonyl
Dimanganese decacarbonyl, which has the chemical formula Mn2(CO)10, is a binary bimetallic carbonyl complex centered around the first row transition metal manganese. The first reported synthesis of Mn2(CO)10 was in 1954 at Linde Air Products Company and was performed by Brimm, Lynch, and Sesny. Their hypothesis about, and synthesis of, dimanganese decacarbonyl was fundamentally guided by the previously known dirhenium decacarbonyl (Re2(CO)10), the heavy atom analogue of Mn2(CO)10. Since its first synthesis, Mn2(CO)10 has been use sparingly as a reagent in the synthesis of other chemical species, but has found the most use as a simple system on which to study fundamental chemical and physical phenomena, most notably, the metal-metal bond. Dimanganese decacarbonyl is also used as a classic example to reinforce fundamental topics in organometallic chemistry like d-electron count, the 18-electron rule, oxidation state, valency, and the isolobal analogy. Synthesis Many procedur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromine
Bromine is a chemical element; it has chemical symbol, symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between those of chlorine and iodine. Isolated independently by two chemists, Carl Jacob Löwig (in 1825) and Antoine Jérôme Balard (in 1826), its name was derived , referring to its sharp and pungent smell. Elemental bromine is very reactive and thus does not occur as a free element in nature. Instead, it can be isolated from colourless soluble crystalline mineral halide Ionic salt, salts analogous to table salt, a property it shares with the other halogens. While it is rather rare in the Earth's crust, the high solubility of the bromide ion (Br) has caused its Bromine cycle, accumulation in the oceans. Commercially the element is easily extracted from brine evaporation ponds, mostly in the United States and Israel. The mass of bromine in the oce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
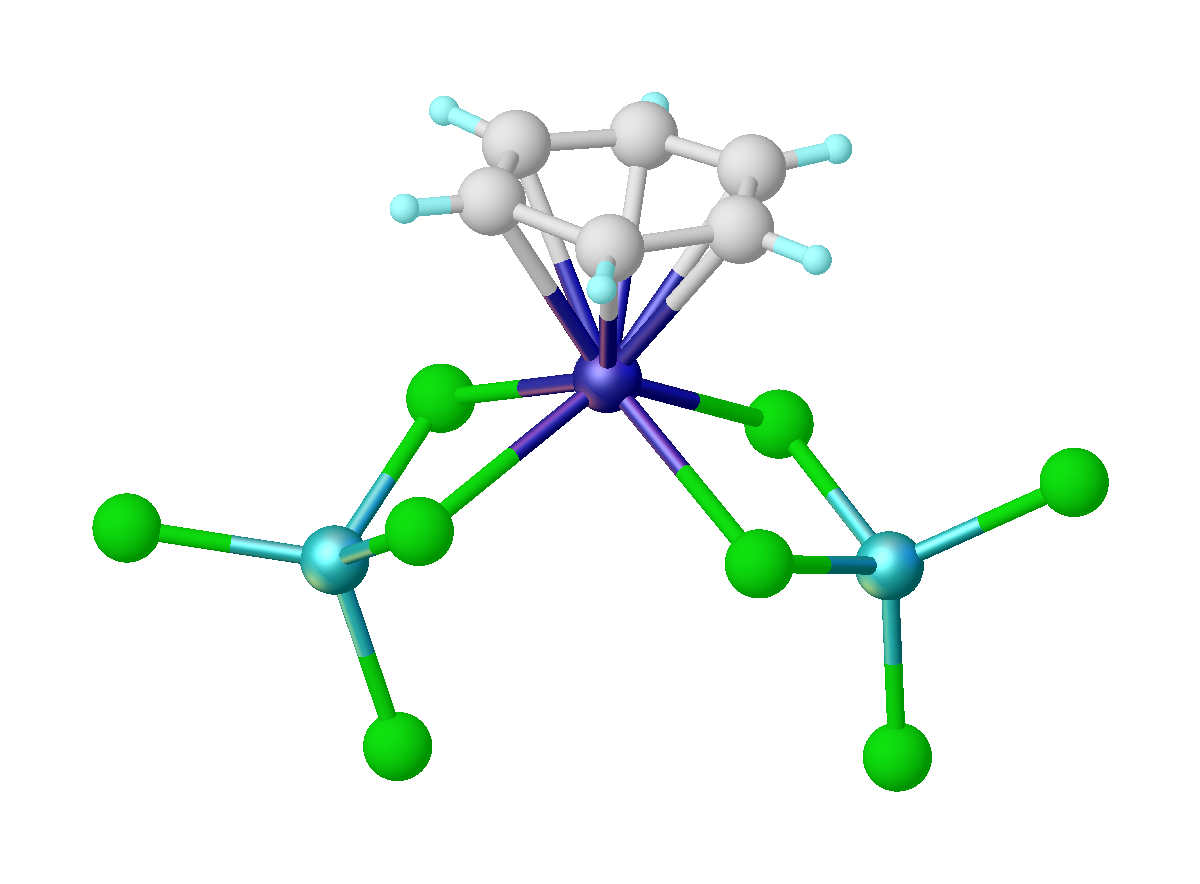
Arene Complex
114px, Structure of Cr(η6-C6H6)2 Metal arene complexes are organometallic compounds of the formula (C6R6)xMLy. Common classes are of the type (C6R6)ML3 and (C6R6)2M. These compounds are reagents in inorganic and organic synthesis. The principles that describe arene complexes extend to related organic ligands such as many heterocycles (e.g. thiophene) and polycyclic aromatic compounds (e.g. naphthalene). Synthesis Fischer–Hafner synthesis Also known as reductive Friedel–Crafts reaction, the Fischer–Hafner synthesis entails treatment of metal chlorides with arenes in the presence of aluminium trichloride and aluminium metal. The method was demonstrated in the 1950s with the synthesis of bis(benzene)chromium by Walter Hafner and his advisor E. O. Fischer. The method has been extended to other metals, e.g. u(C6Me6)2sup>2+. In this reaction, the AlCl3 serves to remove chloride from the metal precursor, and the Al metal functions as the reductant. The Fischer-Hafner synthe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organomanganese Compounds
Organomanganese chemistry is the chemistry of organometallic compounds containing a carbon to manganese chemical bond. In a 2009 review, Cahiez et al. argued that as manganese is cheap and benign (only iron performs better in these aspects), organomanganese compounds have potential as chemical reagents, although currently they are not widely used as such despite extensive research. Synthesis Organomanganese compounds were first reported in 1937 by Gilman and Bailee who described the reaction of phenyllithium and manganese(II) iodide to form phenylmanganese iodide (PhMnI) and diphenylmanganese (Ph2Mn). Following this precedent, other organomanganese halides can be obtained by alkylation of manganese(II) chloride, manganese(II) bromide, and manganese(II) iodide. Manganese iodide is attractive because the anhydrous compound can be prepared in situ from manganese and iodine in ether. Typical alkylating agents are organolithium or organomagnesium compounds: : : A variety of organoman ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonyl Complexes
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such as aldehydes, ketones and carboxylic acid), as part of many larger functional groups. A compound containing a carbonyl group is often referred to as a carbonyl compound. The term carbonyl can also refer to carbon monoxide as a ligand in an inorganic or organometallic complex (a metal carbonyl, e.g. nickel carbonyl). The remainder of this article concerns itself with the organic chemistry definition of carbonyl, such that carbon and oxygen share a double bond. Carbonyl compounds In organic chemistry, a carbonyl group characterizes the following types of compounds: Other organic carbonyls are urea and the carbamates, the derivatives of acyl chlorides, chloroformates and phosgene, carbonate esters, thioesters, lactones, lactams, hydroxamates, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |