|
Magnesium Nitrate
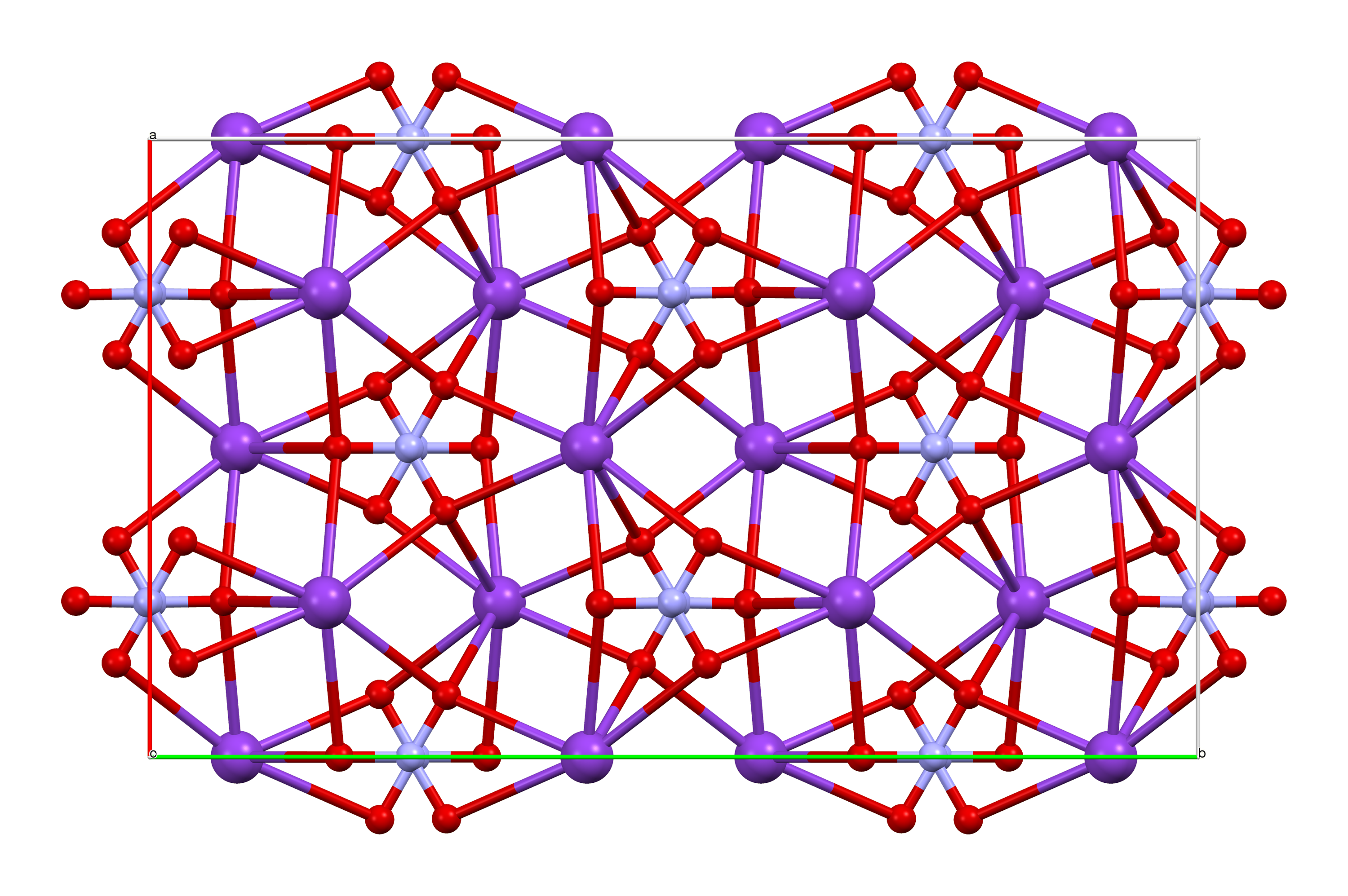
Magnesium nitrate refers to inorganic compounds with the formula Mg(NO3)2(H2O)x, where x = 6, 2, and 0. All are white solids. The anhydrous material is hygroscopic, quickly forming the hexahydrate upon standing in air. All of the salts are very soluble in both water and ethanol. Occurrence, preparation, structure Being highly water-soluble, magnesium nitrate occurs naturally only in Mining, mines and caverns as ''nitromagnesite'' (hexahydrate form).Mindat, http://www.mindat.org/min-2920.html The magnesium nitrate used in commerce is made by the reaction of nitric acid and various magnesium salts. Use The principal use is as a dehydrating agent in the preparation of concentrated nitric acid. Its fertilizer grade has 10.5% nitrogen and 9.4% magnesium, so it is listed as Labeling of fertilizers#Macronutrient fertilizers, 10.5-0-0 + 9.4% Mg. Fertilizer blends containing magnesium nitrate also have ammonium nitrate, calcium nitrate, potassium nitrate and micronutrients in most ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitromagnesite
Magnesium nitrate refers to inorganic compounds with the formula Mg(NO3)2(H2O)x, where x = 6, 2, and 0. All are white solids. The anhydrous material is hygroscopic, quickly forming the hexahydrate upon standing in air. All of the salts are very soluble in both water and ethanol. Occurrence, preparation, structure Being highly water-soluble, magnesium nitrate occurs naturally only in mines and caverns as ''nitromagnesite'' (hexahydrate form).Mindat, http://www.mindat.org/min-2920.html The magnesium nitrate used in commerce is made by the reaction of nitric acid and various magnesium salts. Use The principal use is as a dehydrating agent in the preparation of concentrated nitric acid. Its fertilizer grade has 10.5% nitrogen and 9.4% magnesium, so it is listed as 10.5-0-0 + 9.4% Mg. Fertilizer blends containing magnesium nitrate also have ammonium nitrate, calcium nitrate, potassium nitrate and micronutrients in most cases; these blends are used in the greenhouse and hydro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
80445-ICSD
8 (eight) is the natural number following 7 and preceding 9. Etymology English ''eight'', from Old English '', æhta'', Proto-Germanic ''*ahto'' is a direct continuation of Proto-Indo-European '' *oḱtṓ(w)-'', and as such cognate with Greek and Latin , both of which stems are reflected by the English prefix oct(o)-, as in the ordinal adjective ''octaval'' or ''octavary'', the distributive adjective is ''octonary''. The adjective ''octuple'' (Latin ) may also be used as a noun, meaning "a set of eight items"; the diminutive '' octuplet'' is mostly used to refer to eight siblings delivered in one birth. The Semitic numeral is based on a root ''*θmn-'', whence Akkadian ''smn-'', Arabic ''ṯmn-'', Hebrew ''šmn-'' etc. The Chinese numeral, written (Mandarin: ''bā''; Cantonese Cantonese is the traditional prestige variety of Yue Chinese, a Sinitic language belonging to the Sino-Tibetan language family. It originated in the city of Guangzhou (formerly known ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), nonmetal, and a potent oxidizing agent that readily forms oxides with most elements as well as with other chemical compound, compounds. Oxygen is abundance of elements in Earth's crust, the most abundant element in Earth's crust, making up almost half of the Earth's crust in the form of various oxides such as water, carbon dioxide, iron oxides and silicates.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. It is abundance of chemical elements, the third-most abundant element in the universe after hydrogen and helium. At standard temperature and pressure, two oxygen atoms will chemical bond, bind covalent bond, covalently to form dioxygen, a colorless and odorless diatomic gas with the chemical formula ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Oxide
Magnesium oxide (MgO), or magnesia, is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium (see also oxide). It has an empirical formula of MgO and consists of a lattice of Mg2+ ions and O2− ions held together by ionic bonding. Magnesium hydroxide forms in the presence of water (MgO + H2O → Mg(OH)2), but it can be reversed by heating it to remove moisture. Magnesium oxide was historically known as magnesia alba (literally, the white mineral from Magnesia), to differentiate it from '' magnesia nigra'', a black mineral containing what is now known as manganese. Related oxides While "magnesium oxide" normally refers to MgO, the compound magnesium peroxide MgO2 is also known. According to evolutionary crystal structure prediction, MgO2 is thermodynamically stable at pressures above 116 GPa (gigapascals), and a semiconducting suboxide Mg3O2 is thermodynamically stable above 500 GPa. Because of its stability, MgO is used as a mod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dehydration Reaction
In chemistry, a dehydration reaction is a chemical reaction that involves the loss of an H2O from the reacting molecule(s) or ion(s). This reaction results in the release of the H2O as water. When the reaction involves the coupling of two molecules into a single molecule it is referred to as a condensation reaction. Dehydration reactions are common processes in the manufacture of chemical compounds as well as naturally occurring within living organisms. The reverse of a dehydration reaction is called a hydration reaction. The reverse of a condensation reaction yielding water is called hydrolysis. Condensation reactions occurring in living organisms Condensation dehydration reactions are fundamental to the existence of life as this type of reaction produces proteins from amino acids, DNA and RNA from nucleotides, fats from fatty acids, and polysaccharides (eg. cellulose, starch, sugar, lactose) from monosaccharides (eg. glucose and fructose). The formation of the pyrophosphat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroponics
Hydroponics is a type of horticulture and a subset of #Passive sub-irrigation, hydroculture which involves growing plants, usually crops or medicinal plants, without soil, by using water-based mineral Plant nutrition, nutrient Solution (chemistry), solutions in an artificial environment. Terrestrial plant, Terrestrial or aquatic plants may grow freely with their roots exposed to the nutritious liquid or the roots may be mechanically supported by an inert medium such as perlite, gravel, or Hydroponics#Substrates (growing support materials), other substrates. Despite inert media, roots can cause changes of the rhizosphere pH and root exudates can affect rhizosphere biology and physiological balance of the nutrient solution when secondary metabolites are produced in plants. Genetically modified plant, Transgenic plants grown hydroponically allow the release of Pharming (genetics)#In plants, pharmaceutical proteins as part of the root exudate into the hydroponic medium. The nutri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Greenhouse
A greenhouse is a structure that is designed to regulate the temperature and humidity of the environment inside. There are different types of greenhouses, but they all have large areas covered with transparent materials that let sunlight pass and block it as heat. The most common materials used in modern greenhouses for walls and roofs are rigid plastic made of polycarbonate, plastic film made of polyethylene, or glass panes. When the inside of a greenhouse is exposed to sunlight, the temperature increases, providing a sheltered environment for plants to grow even in cold weather. The terms greenhouse, glasshouse, and hothouse are often used interchangeably to refer to buildings used for cultivating plants. The specific term used depends on the material and heating system used in the building. Nowadays, greenhouses are more commonly constructed with a variety of materials, such as wood and polyethylene plastic. A glasshouse, on the other hand, is a traditional type of greenhouse ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Micronutrients
Micronutrients are essential chemicals required by organisms in small quantities to perform various biogeochemical processes and regulate physiological functions of cells and organs. By enabling these processes, micronutrients support the health of organisms throughout life. For humans, micronutrients typically take one of three forms: vitamins, trace elements, and dietary minerals. Human micronutrient requirements are in amounts generally less than 100 milligrams per day, whereas macronutrients are required in gram quantities daily. Deficiencies in micronutrient intake commonly result in malnutrition. In ecosystems, micronutrients most commonly take the form of trace elements such as iron, strontium, and manganese. Micronutrient abundance in the environment greatly influences biogeochemical cycles at the microbial level which large ecological communities rely on to survive. For example, marine primary producers are reliant upon bioavailable dissolved iron for photosyn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Nitrate
Potassium nitrate is a chemical compound with a sharp, salty, bitter taste and the chemical formula . It is a potassium salt of nitric acid. This salt consists of potassium cations and nitrate anions , and is therefore an alkali metal nitrate. It occurs in nature as a mineral, niter (or ''nitre'' outside the United States). It is a source of nitrogen, and nitrogen was named after niter. Potassium nitrate is one of several nitrogen-containing compounds collectively referred to as saltpetre (or saltpeter in the United States). Major uses of potassium nitrate are in fertilizers, tree stump removal, rocket propellants and fireworks. It is one of the major constituents of traditional gunpowder (black powder). In processed meats, potassium nitrate reacts with hemoglobin and myoglobin generating a red color. Etymology Nitre, or potassium nitrate, because of its early and global use and production, has many names. As for nitrate, Egyptian and Hebrew words for it had the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Nitrate
Calcium nitrate are inorganic compounds with the formula Ca(NO3)2(H2O)x. The anhydrous compound, which is rarely encountered, absorbs moisture from the air to give the tetrahydrate. Both anhydrous and hydrated forms are colourless salts. Hydrated calcium nitrate, also called ''Norgessalpeter'' (Norwegian salpeter), is mainly used as a component in fertilizers, but it has other applications. Nitrocalcite is the name for a mineral which is a hydrated calcium nitrate that forms as an efflorescence where manure contacts concrete or limestone in a dry environment as in stables or caverns. A variety of related salts are known including calcium ammonium nitrate decahydrate and calcium potassium nitrate decahydrate. Production and reactivity Norgessalpeter was synthesized at Notodden, Norway in 1905 by the Birkeland–Eyde process. Most of the world's calcium nitrate is now made in Porsgrunn. It is produced by treating limestone with nitric acid, followed by neutralization with ammonia: ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium Nitrate
Ammonium nitrate is a chemical compound with the formula . It is a white crystalline salt consisting of ions of ammonium and nitrate. It is highly soluble in water and hygroscopic as a solid, but does not form hydrates. It is predominantly used in agriculture as a high-nitrogen fertilizer. Its other major use is as a component of explosive mixtures used in mining, quarrying, and civil construction. It is the major constituent of ANFO, an industrial explosive which accounts for 80% of explosives used in North America; similar formulations have been used in improvised explosive devices. Many countries are phasing out its use in consumer applications due to concerns over its potential for misuse.Ammonium nitrate sold by ton as U.S. regulati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Labeling Of Fertilizers
Many countries have standardized the labeling of fertilizers to indicate their contents of major nutrients. The most common labeling convention, the NPK or N-P-K label, shows the amounts of the chemical elements nitrogen, phosphorus, and potassium. Common labeling conventions The NPK analysis label Fertilizers are usually labeled with three numbers, as in 18-20-10, indicating the relative content of the primary macronutrients nitrogen (N), phosphorus (P), and potassium (K), respectively. More precisely, the first number ("N value") is the percentage of elemental nitrogen by weight in the fertilizer; that is, the mass fraction of nitrogen times 100. The second number ("P value") is the percentage by weight of phosphorus pentoxide . The third number ("K value") is the equivalent content of potassium oxide . For example, a 15-13-20 fertilizer would contain 15% by weight of nitrogen, 13% by weight of , 20% by weight of , and 52% of some inert ingredient. Other labeling conv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |