|
MYO10
Myosin X, also known as MYO10, is a protein that in humans is encoded by the ''MYO10'' gene. Myo10 is an actin-based motor protein that can localize to the tips of the finger-like cellular protrusions known as filopodia. Myo10 is broadly expressed in mammalian tissues, although at relatively low levels. Studies with knockout mice demonstrate that Myo10 has important functions in embryonic processes such as neural tube closure and eye development. Myo10 also has important functions in cancer invasion and growth. Myo10 should not be confused with Myh10, which encodes the heavy chain of the class II myosin known as non-muscle myosin 2b. Structure and function The human ''MYO10'' gene spans ~274 kb and is located on chromosome 5 band 5p15.1 (GRCh Ensembl release 89). It produces a full-length RNA transcript with 41 exons encoding a MYO10 heavy chain whose deduced sequence has 2058 amino acids and a predicted molecular weight of ~237 kDa. Like many motor proteins, the full-lengt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pleckstrin Homology Domain
Pleckstrin homology domain (PH domain) or (PHIP) is a protein domain of approximately 120 amino acids that occurs in a wide range of proteins involved in intracellular signaling or as constituents of the cytoskeleton. This domain can bind phosphatidylinositol lipids within biological membranes (such as phosphatidylinositol (3,4,5)-trisphosphate and phosphatidylinositol (4,5)-bisphosphate), and proteins such as the βγ-subunits of heterotrimeric G proteins, and protein kinase C. Through these interactions, PH domains play a role in recruiting proteins to different membranes, thus targeting them to appropriate cellular compartments or enabling them to interact with other components of the signal transduction pathways. Lipid binding specificity Individual PH domains possess specificities for phosphoinositides phosphorylated at different sites within the inositol ring, e.g., some bind phosphatidylinositol (4,5)-bisphosphate but not phosphatidylinositol (3,4,5)-trisphosphate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid resid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Filozoa
The Filozoa are a monophyletic grouping within the Opisthokonta. They include animals and their nearest unicellular relatives (those organisms which are more closely related to animals than to fungi or Mesomycetozoa). Three groups are currently assigned to the clade Filozoa: * Group Filasterea - recently erected to house the genera ''Ministeria'' and ''Capsaspora'' * Group Choanoflagellatea - collared flagellates * Kingdom Animalia - the animals proper Etymology From Latin ''filum'' meaning "thread" and Greek ''zōion'' meaning "animal". Phylogeny A phylogenetic tree of Filozoa and its most closely related clades: Characteristics The ancestral opisthokont cell is assumed to have possessed slender filose (thread-like) projections or 'tentacles'. In some opisthokonts (Mesomycetozoa and ''Corallochytrium'') these were lost. They are retained in Filozoa, where they are simple and non-tapering, with a rigid core of actin bundles (contrasting with the flexible, tapering and b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dictyostelium
''Dictyostelium'' is a genus of single- and multi-celled eukaryotic, phagotrophic bacterivores. Though they are Protista and in no way fungal, they traditionally are known as "slime molds". They are present in most terrestrial ecosystems as a normal and often abundant component of the soil microflora, and play an important role in the maintenance of balanced bacterial populations in soils. The genus ''Dictyostelium'' is in the order Dictyosteliida, the so-called cellular slime molds or social amoebae. In turn the order is in the infraphylum Mycetozoa. Members of the order are Protista of great theoretical interest in biology because they have aspects of both unicellularity and multicellularity. The individual cells in their independent phase are common on organic detritus or in damp soils and caves. In this phase they are amoebae. Typically, the amoebal cells grow separately and wander independently, feeding mainly on bacteria. However, they interact to form multi-cellu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stereocilia (inner Ear)
In the inner ear, stereocilia are the mechanosensing organelles of hair cells, which respond to fluid motion in numerous types of animals for various functions, including hearing and balance. They are about 10–50 micrometers in length and share some similar features of microvilli.Caceci, T. VM8054 Veterinary Histology: Male Reproductive System. http://education.vetmed.vt.edu/Curriculum/VM8054/Labs/Lab27/Lab27.htm (accessed 2/16/06). The hair cells turn the fluid pressure and other mechanical stimuli into electric stimuli via the many microvilli that make up stereocilia rods.Alberts, B., Johnson, A., Lewis, J., Raff, M., Roberts, K. and Walter, P. (2002) The Molecular Biology of the Cell. Garland Science Textbooks. Stereocilia exist in the auditory and vestibular systems. Morphology Resembling hair-like projections, the stereocilia are arranged in bundles of 30-300. Within the bundles the stereocilia are often lined up in several rows of increasing height, similar to a stai ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microvillus
Microvilli (singular: microvillus) are microscopic cellular membrane protrusions that increase the surface area for diffusion and minimize any increase in volume, and are involved in a wide variety of functions, including absorption, secretion, cellular adhesion, and mechanotransduction. Structure Microvilli are covered in plasma membrane, which encloses cytoplasm and microfilaments. Though these are cellular extensions, there are little or no cellular organelles present in the microvilli. Each microvillus has a dense bundle of cross-linked actin filaments, which serves as its structural core. 20 to 30 tightly bundled actin filaments are cross-linked by bundling proteins fimbrin (or plastin-1), villin and espin to form the core of the microvilli. In the enterocyte microvillus, the structural core is attached to the plasma membrane along its length by lateral arms made of myosin 1a and Ca2+ binding protein calmodulin. Myosin 1a functions through a binding site for filamentous ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MYO6
Unconventional myosin-VI, is a protein that in humans is coded for by ''MYO6''. Unconventional myosin-VI is a myosin molecular motor involved in intracellular vesicle and organelle transport. Structure Human myosin-VI contains a N-terminal myosin head domain (residues 59–759), two coiled coil motifs (residues 902–984 and 986–1009 respectively), and a C-terminal myosin VI cargo binding domain (residues 1177–1267). Function Unconventional myosin-VI is unique because it travels in the opposite direction of other myosins, towards the negative end of actin filaments. Myosin-VI follows the same structure as other myosin but with two unique "inserts" allowing for its diversified properties. One insert is called the "reverse gear" and is responsible for its movement towards the negative end of actin filaments. The reverse gear is located on the neck region of the myosin and acts as a reorienting device for the lever arm to move backwards after myosin movement. The second inse ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NEO1
Neogenin is a protein that in humans is encoded by the ''NEO1'' gene. Interactions NEO1 has been shown to interact Advocates for Informed Choice, dba interACT or interACT Advocates for Intersex Youth, is a 501(c)(3) nonprofit organization using innovative strategies to advocate for the legal and human rights of children with intersex traits. The organizati ... with PTK2. Also Neogenin receptor is pointed as a component of the mechanisms that determine skeletal cell fusion via RGMa (repulsive guidance molecule a) binding. References Further reading * * * * * * * * * * Human proteins {{gene-15-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deleted In Colorectal Cancer
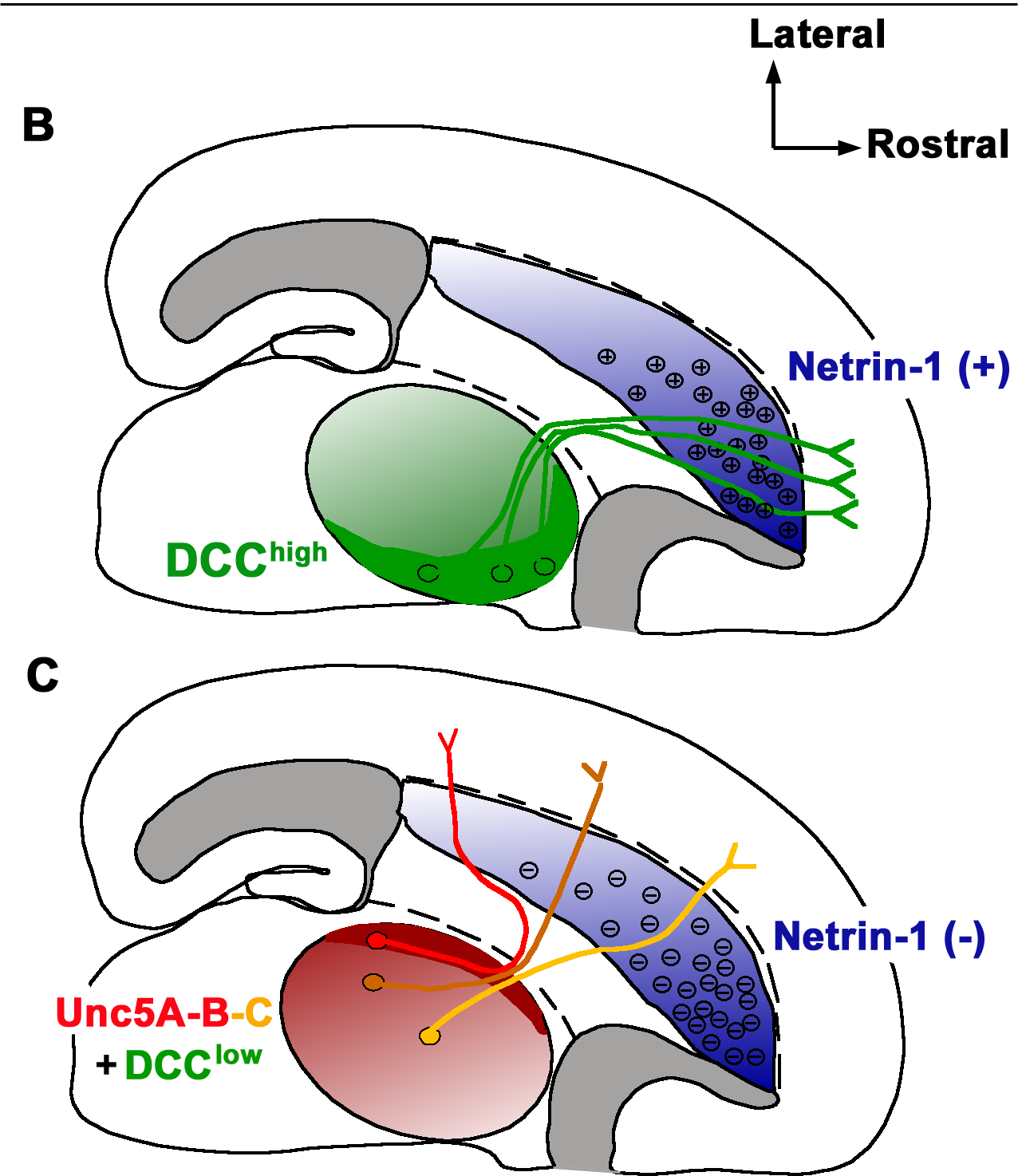
Netrin receptor DCC, also known as DCC, or colorectal cancer suppressor is a protein which in humans is encoded by the ''DCC'' gene. DCC has long been implicated in colorectal cancer and its previous name was ''Deleted in colorectal carcinoma''. Netrin receptor DCC is a single transmembrane receptor. Since it was first discovered in a colorectal cancer study in 1990, ''DCC'' has been the focus of a significant amount of research. ''DCC'' held a controversial place as a tumour suppressor gene for many years, and is well known as an axon guidance receptor that responds to netrin-1. More recently DCC has been characterized as a #DCC as a dependence receptor, dependence receptor, and many hypotheses have been put forward that have revived interest in ''DCCs candidacy as a tumour suppressor gene, as it may be a ligand-dependent suppressor that is frequently epigenetically silenced. Background Early studies of colorectal tumours found that allelic deletions of segments of chromosome ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Netrin
Netrins are a class of proteins involved in axon guidance. They are named after the Sanskrit word "netr", which means "one who guides". Netrins are genetically conserved across nematode worms, fruit flies, frogs, mice, and humans. Structurally, netrin resembles the extracellular matrix protein laminin. Netrins are chemotropic; a growing axon will either move towards or away from a higher concentration of netrin. Though the detailed mechanism of axon guidance is not fully understood, it is known that netrin attraction is mediated through UNC-40/DCC cell surface receptors and repulsion is mediated through UNC-5 receptors. Netrins also act as growth factors, encouraging cell growth activities in target cells. Mice deficient in netrin fail to form the hippocampal comissure or the corpus callosum. A proposed model for netrin activity in the spinal column of developing human embryos is that netrins are released by the floor plate and then are picked up by receptor protei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Integrin
Integrins are transmembrane receptors that facilitate cell-cell and cell-extracellular matrix (ECM) adhesion. Upon ligand binding, integrins activate signal transduction pathways that mediate cellular signals such as regulation of the cell cycle, organization of the intracellular cytoskeleton, and movement of new receptors to the cell membrane. The presence of integrins allows rapid and flexible responses to events at the cell surface (''e.g''. signal platelets to initiate an interaction with coagulation factors). Several types of integrins exist, and one cell generally has multiple different types on its surface. Integrins are found in all animals while integrin-like receptors are found in plant cells. Integrins work alongside other proteins such as cadherins, the immunoglobulin superfamily cell adhesion molecules, selectins and syndecans, to mediate cell–cell and cell–matrix interaction. Ligands for integrins include fibronectin, vitronectin, collagen and laminin. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microtubule
Microtubules are polymers of tubulin that form part of the cytoskeleton and provide structure and shape to eukaryotic cells. Microtubules can be as long as 50 micrometres, as wide as 23 to 27 nm and have an inner diameter between 11 and 15 nm. They are formed by the polymerization of a dimer of two globular proteins, alpha and beta tubulin into protofilaments that can then associate laterally to form a hollow tube, the microtubule. The most common form of a microtubule consists of 13 protofilaments in the tubular arrangement. Microtubules play an important role in a number of cellular processes. They are involved in maintaining the structure of the cell and, together with microfilaments and intermediate filaments, they form the cytoskeleton. They also make up the internal structure of cilia and flagella. They provide platforms for intracellular transport and are involved in a variety of cellular processes, including the movement of secretory vesicles, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |