|
List Of Liquid–liquid Phase Separation Databases
Liquid-liquid phase separation (LLPS) is well defined in the Biomolecular condensate page. LLPS databases cover different aspects of LLPS phenomena, ranging from cellular location of the Membraneless Organelles (MLOs) to the role of a particular protein/region forming the condensate state. These databases contain manually curated data supported by experimental evidence in the literature and can include related features as presence of protein disorder, low complexity, post-translational modifications, experimental details, phase diagrams, among others. See also *Biomolecular condensate * MobiDB database *Intrinsically disordered proteins In molecular biology, an intrinsically disordered protein (IDP) is a protein that lacks a fixed or ordered protein tertiary structure, three-dimensional structure, typically in the absence of its macromolecular interaction partners, such as other ... * DisProt database References {{DEFAULTSORT:List of LLPS-Liquid liquid phase separat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase Separation
Phase separation is the creation of two distinct Phase (matter), phases from a single homogeneous mixture. The most common type of phase separation is between two immiscible liquids, such as oil and water. This type of phase separation is known as liquid-liquid equilibrium. Colloids are formed by phase separation, though not all phase separations forms colloids - for example oil and water can form separated layers under gravity rather than remaining as microscopic droplets in suspension. A common form of spontaneous phase separation is termed spinodal decomposition; it is described by the Cahn–Hilliard equation. Regions of a phase diagram in which phase separation occurs are called miscibility gaps. There are two boundary curves of note: the binodal, binodal coexistence curve and the spinodal, spinodal curve. On one side of the binodal, mixtures are absolutely stable. In between the binodal and the spinodal, mixtures may be metastable: staying mixed (or unmixed) absent some ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biomolecular Condensate
In biochemistry, biomolecular condensates are a class of membrane-less organelles and organelle subdomains, which carry out specialized functions within the cell. Unlike many organelles, biomolecular condensate composition is not controlled by a bounding membrane. Instead, condensates can form and maintain organization through a range of different processes, the most well-known of which is phase separation of proteins, RNA, and other biopolymers into either colloidal emulsions, gels, liquid crystals, solid crystals, or aggregates within cells. History Micellar theory The micellar theory of Carl Nägeli was developed from his detailed study of starch granules in 1858. Amorphous substances such as starch and cellulose were proposed to consist of building blocks, packed in a loosely crystalline array to form what he later termed "micelles". Water could penetrate between the micelles, and new micelles could form in the interstices between old micelles. The swelling of st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MobiDB
In molecular biology, MobiDB is a curated biological database designed to offer a centralized resource for annotations of intrinsic protein disorder. Protein disorder is a structural feature characterizing a large number of proteins with prominent members known as intrinsically unstructured (or disordered) proteins. The database features three levels of annotation: manually curated, indirect and predicted. By combining different data sources of protein disorder into a consensus annotation, MobiDB aims at giving the best possible picture of the "disorder landscape" of a given protein of interest. MobiDB data sources Curated data and additional annotation Curated data for MobiDB is obtained from DisProt database giving information and disorder annotation manually extracted from literature. In order to complement disorder annotation, MobiDB features additional annotations from external sources: * UniProt: Annotations from the UniProt database include organism, subcellular loca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intrinsically Disordered Proteins
In molecular biology, an intrinsically disordered protein (IDP) is a protein that lacks a fixed or ordered protein tertiary structure, three-dimensional structure, typically in the absence of its macromolecular interaction partners, such as other proteins or RNA. IDPs range from fully unstructured to partially structured and include random coil, molten globule-like Protein aggregation, aggregates, or flexible linkers in large multi-Protein domain, domain proteins. They are sometimes considered as a separate class of proteins along with globular protein, globular, fibrous protein, fibrous and membrane proteins. IDPs are a very large and functionally important class of proteins. They are most numerous in Eukaryote, eukaryotes, with an estimated 30-40% of residues in the eukaryotic proteome located in disordered regions. Disorder is present in around 70% of proteins, either in the form of disordered tails or flexible linkers. Proteins can also be entirely disordered and lack a define ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
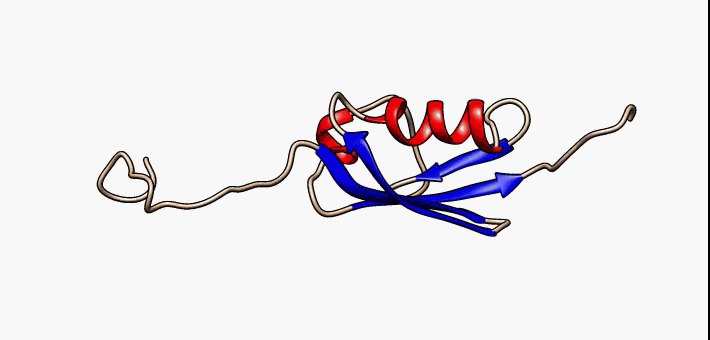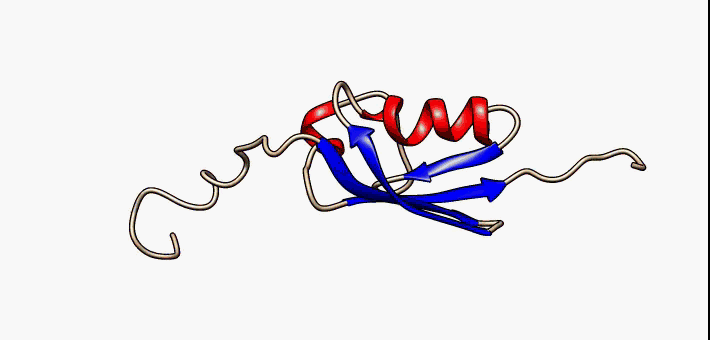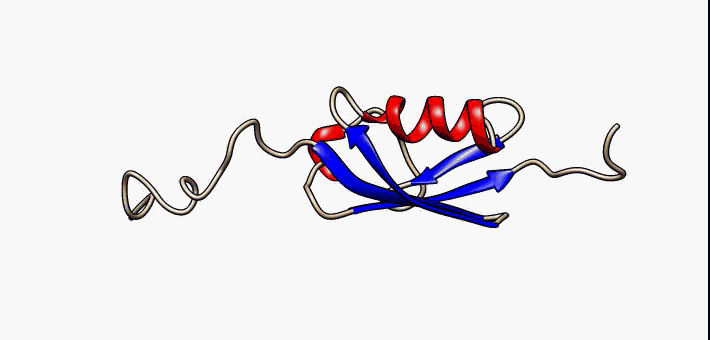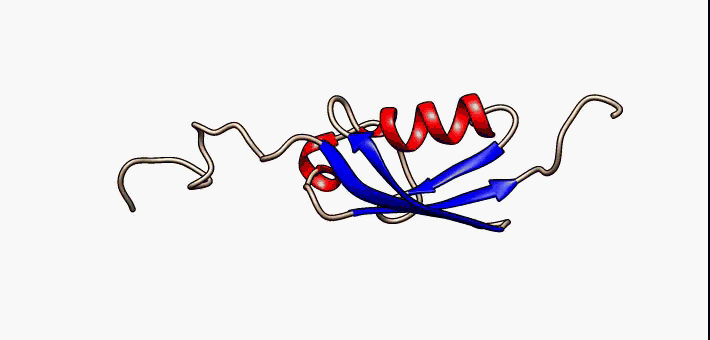
Protein Structure
Protein structure is the three-dimensional arrangement of atoms in an amino acid-chain molecule. Proteins are polymers specifically polypeptides formed from sequences of amino acids, which are the monomers of the polymer. A single amino acid monomer may also be called a ''residue'', which indicates a repeating unit of a polymer. Proteins form by amino acids undergoing condensation reactions, in which the amino acids lose one water molecule per reaction in order to attach to one another with a peptide bond. By convention, a chain under 30 amino acids is often identified as a peptide, rather than a protein. To be able to perform their biological function, proteins fold into one or more specific spatial conformations driven by a number of non-covalent interactions, such as hydrogen bonding, ionic interactions, Van der Waals forces, and hydrophobic packing. To understand the functions of proteins at a molecular level, it is often necessary to determine their three-dimensiona ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Structural Bioinformatics Software
A structure is an arrangement and organization of interrelated elements in a material object or system, or the object or system so organized. Material structures include man-made objects such as buildings and machines and natural objects such as biological organisms, minerals and chemicals. Abstract structures include data structures in computer science and musical form. Types of structure include a hierarchy (a cascade of one-to-many relationships), a network featuring many-to-many links, or a lattice featuring connections between components that are neighbors in space. Load-bearing Buildings, aircraft, skeletons, anthills, beaver dams, bridges and salt domes are all examples of load-bearing structures. The results of construction are divided into buildings and non-building structures, and make up the infrastructure of a human society. Built structures are broadly divided by their varying design approaches and standards, into categories including building structur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteomics
Proteomics is the large-scale study of proteins. Proteins are vital macromolecules of all living organisms, with many functions such as the formation of structural fibers of muscle tissue, enzymatic digestion of food, or synthesis and replication of DNA. In addition, other kinds of proteins include antibodies that protect an organism from infection, and hormones that send important signals throughout the body. The proteome is the entire set of proteins produced or modified by an organism or system. Proteomics enables the identification of ever-increasing numbers of proteins. This varies with time and distinct requirements, or stresses, that a cell or organism undergoes. Proteomics is an interdisciplinary domain that has benefited greatly from the genetic information of various genome projects, including the Human Genome Project. It covers the exploration of proteomes from the overall level of protein composition, structure, and activity, and is an important component of function ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |