|
List Of Schedule V Controlled Substances (U.S.)
This is the list of Schedule V controlled substances in the United States as defined by the Controlled Substances Act. The following findings are required for substances to be placed in this schedule: retrieved October 7, 2007 # The drug or other substance has a low potential for abuse relative to the drugs or other substances in schedule IV. # The drug or other substance has a currently accepted medical use in treatment in the United States. # Abuse of the drug or other substance may lead to limited physical dependence or psychological dependence relative to the drugs or other substances in schedule IV. The complete list of Schedule V substances is as follows. The Administrative Controlled Substances Code Number and Federal Register citation for each substance is included. Opiates and opioids Stimulants Others See also * List of Schedule I controlled substances (U.S.) * List of Schedule II controlled substances (U.S.) This is the list of Schedule II controlled substances i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Controlled Substances Act
The Controlled Substances Act (CSA) is the statute establishing federal government of the United States, federal drug policy of the United States, U.S. drug policy under which the manufacture, importation, possession, use, and distribution of certain substances is regulated. It was passed by the 91st United States Congress as Title II of the Comprehensive Drug Abuse Prevention and Control Act of 1970 and signed into law by President Richard Nixon. The Act also served as the national implementing legislation for the Single Convention on Narcotic Drugs. The legislation created five schedules (classifications), with varying qualifications for a substance to be included in each. Two federal agencies, the Drug Enforcement Administration (DEA) and the Food and Drug Administration (FDA), determine which substances are added to or removed from the various schedules, although the statute passed by Congress created the initial listing. Congress has sometimes scheduled other substances t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
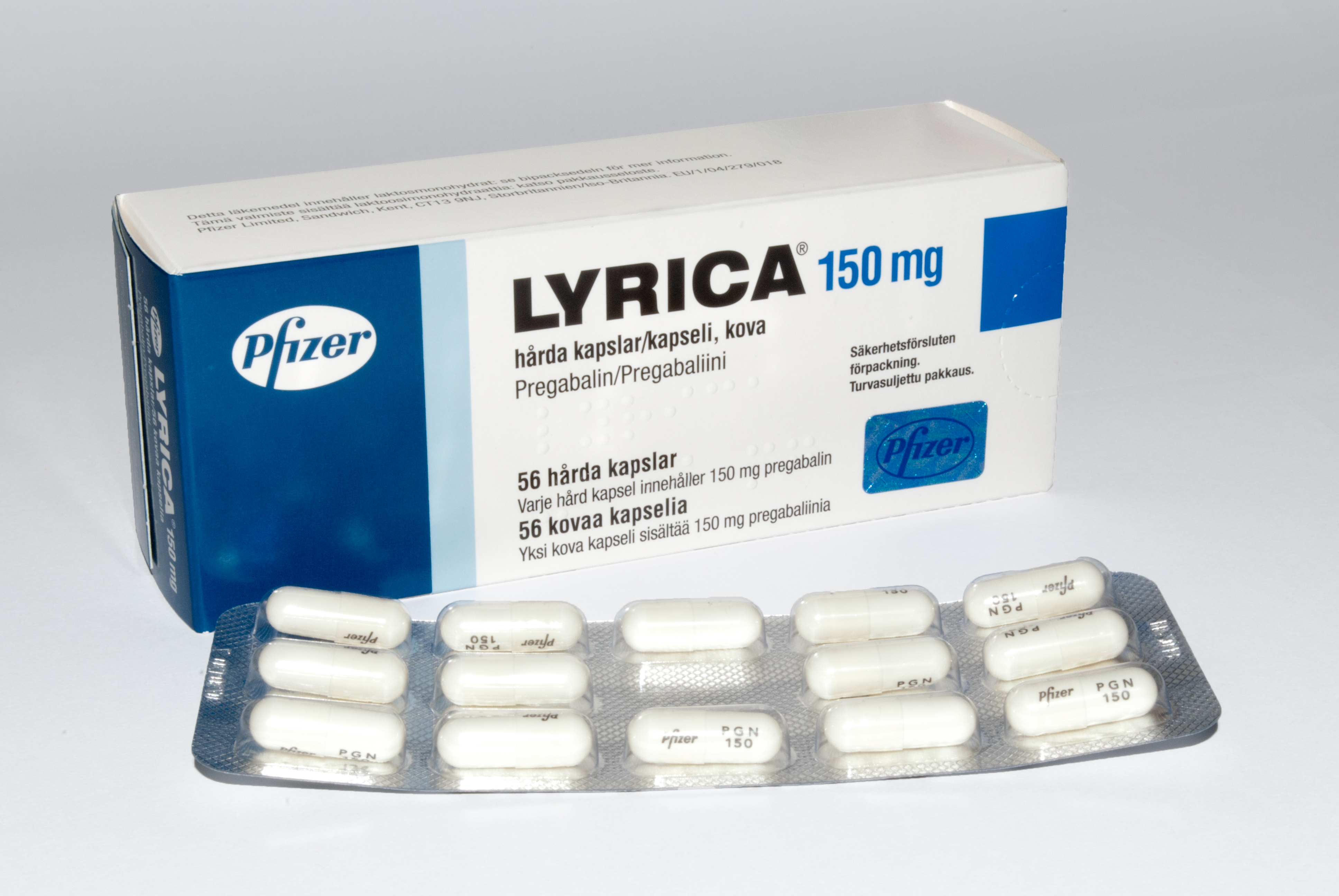
Lyrica 150mg Box In Finland 20110618
Pregabalin, sold under the brand name Lyrica among others, is an anticonvulsant, analgesic, and anxiolytic amino acid medication used to treat epilepsy, neuropathic pain, fibromyalgia, restless legs syndrome, opioid withdrawal, generalized anxiety disorder (GAD), and shingles. Pregabalin also has antiallodynic properties. Its use in epilepsy is as an add-on therapy for partial seizures. When used before surgery, it reduces pain but results in greater sedation and visual disturbances. It is taken by mouth. Common side effects can include headache, dizziness, sleepiness, euphoria, confusion, trouble with memory, poor coordination, dry mouth, problems with vision, and weight gain. Serious side effects may include angioedema, kidney damage and drug misuse. As with all other drugs approved by the FDA for treating epilepsy, the pregabalin labeling warns of an increased suicide risk when combined with other drugs. When pregabalin is taken at high doses over a long period of time, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of Schedule III Controlled Substances (U
A list is a set of discrete items of information collected and set forth in some format for utility, entertainment, or other purposes. A list may be memorialized in any number of ways, including existing only in the mind of the list-maker, but lists are frequently written down on paper, or maintained electronically. Lists are "most frequently a tool", and "one does not ''read'' but only ''uses'' a list: one looks up the relevant information in it, but usually does not need to deal with it as a whole".Lucie Doležalová,The Potential and Limitations of Studying Lists, in Lucie Doležalová, ed., ''The Charm of a List: From the Sumerians to Computerised Data Processing'' (2009). Purpose It has been observed that, with a few exceptions, "the scholarship on lists remains fragmented". David Wallechinsky, a co-author of ''The Book of Lists'', described the attraction of lists as being "because we live in an era of overstimulation, especially in terms of information, and lists help us ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pregabalin
Pregabalin, sold under the brand name Lyrica among others, is an anticonvulsant, analgesic, and anxiolytic amino acid medication used to treat epilepsy, neuropathic pain, fibromyalgia, restless legs syndrome, opioid withdrawal, generalized anxiety disorder (GAD), and shingles. Pregabalin also has allodynia, antiallodynic properties. Its use in epilepsy is as an add-on therapy for partial seizures. When used before surgery, it reduces pain but results in greater sedation and visual disturbances. It is taken oral administration, by mouth. Common side effects can include headache, dizziness, somnolence, sleepiness, euphoria, confusion, trouble with memory, Ataxia, poor coordination, dry mouth, problems with vision, and weight gain. Serious side effects may include angioedema, kidney damage and drug misuse. As with all other drugs approved by the FDA for treating epilepsy, the pregabalin labeling warns of an increased suicide risk when combined with other drugs. When pregabalin i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lasmiditan
Lasmiditan, sold under the brand name Reyvow, is a medication used for the acute (active but short-term) treatment of migraine with or without aura (a sensory phenomenon or visual disturbance) in adults. It is not useful for prevention. It is taken by mouth. Common side effects include sleepiness, dizziness, tiredness, and numbness. Lasmiditan was approved in the United States in October 2019 and became available in February 2020. It was developed by Eli Lilly. The U.S. Food and Drug Administration (FDA) considers it to be a first-in-class medication. Pharmacology Mechanism of action Lasmiditan is a serotonin receptor agonist that, like the unsuccessful LY-334,370, selectively binds to the 5-HT1F receptor subtype. A number of triptans have been shown to act on this subtype as well, but only after their affinity for 5-HT1B and 5-HT1D has been made responsible for their anti-migraine activity. The lack of affinity for these receptors might result in fewer side effects re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lacosamide
Lacosamide, sold under the brand name Vimpat among others, is a medication used for the treatment of partial-onset seizures and primary generalized tonic-clonic seizures. It is used by mouth or intravenously. It is available as a generic medication. Medical uses Lacosamide is indicated for the treatment of partial-onset seizures and adjunctive therapy in the treatment of primary generalized tonic-clonic seizures. Off-label use As with other anti-epileptic drugs (AEDs), lacosamide may have a variety of off-label uses, including for pain management and treatment of mental health disorders. Lacosamide and other AEDs have been used off-label in the management of bipolar disorder, cocaine addiction, dementia, depression, diabetic peripheral neuropathy, fibromyalgia, headache, hiccups, Huntington's disease, mania, migraine, obsessive-compulsive disorder, panic disorder, restless leg syndrome, and tinnitus. Combinations of AEDs are often employed for seizure reduction. Studies a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ganaxolone
Ganaxolone, sold under the brand name Ztalmy, is a medication used to treat seizures in people with cyclin-dependent kinase-like 5 (CDKL5) deficiency disorder. Ganaxolone is a neuroactive steroid gamma-aminobutyric acid (GABA) A receptor positive modulator. The most common side effects of treatment with ganaxolone include somnolence (sleepiness), fever, excessive saliva or drooling, and seasonal allergy. Ganaxolone was approved for medical use in the United States in March 2022, and in the European Union in July 2023. The US Food and Drug Administration (FDA) considers it to be a first-in-class medication. Medical uses Ganaxolone is indicated for the treatment of seizures associated with cyclin-dependent kinase-like 5 (CDKL5) deficiency disorder. Pharmacology Mechanism of action The exact mechanism of action for ganaxolone is unknown; however, results from animal studies suggest that it acts by blocking seizure propagation and elevating seizure thresholds. Ganaxolone ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ezogabine
Retigabine ( INN) or ezogabine ( USAN) is an anticonvulsant used as an adjunctive treatment for partial epilepsies in treatment-experienced adult patients. The drug was developed by Valeant Pharmaceuticals and GlaxoSmithKline. It was approved by the European Medicines Agency under the trade name Trobalt on March 28, 2011, and by the United States Food and Drug Administration (FDA), under the trade name Potiga, on June 10, 2011. Production was discontinued in June 2017. Retigabine works primarily as a potassium channel opener—that is, by activating a certain family of voltage-gated potassium channels in the brain. This mechanism of action is unique among antiepileptic drugs, and may hold promise for the treatment of other neurologic conditions, including tinnitus, migraine and neuropathic pain. The manufacturer withdrew retigabine from clinical use in 2017. Adverse effects The adverse effects found in the Phase II trial mainly affected the central nervous system, and appeared ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cenobamate
Cenobamate, sold under the brand names Xcopri (US) and Ontozry (EU), is a medication used for the treatment of partial-onset seizures, a kind of epilepsy, in adults. It is taken by mouth. Cenobamate was approved for medical use in the United States in November 2019, and placed in Schedule V of the Controlled Substances Act in March 2020. Cenobamate was authorized for medical use in the European Union in March 2021, approved for use in the UK in December 2021, and approved for use in Canada in June 2023. Medical uses In the United States, cenobamate is indicated for the treatment of partial-onset seizures in adults. In the European Union, it is indicated for the adjunctive treatment of focal-onset seizures with or without secondary generalization in adults with epilepsy who have not been adequately controlled despite a history of treatment with at least two anti-epileptic medications. In the UK, it is used as an add-on treatment, after at least 1 other add-on treatme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Brivaracetam
Brivaracetam, sold under the brand name Briviact among others, is a chemical analog of levetiracetam, a racetam derivative with anticonvulsant (antiepileptic) properties. It has been approved since 2016. It is marketed by the pharmaceutical company UCB. It is used to treat partial-onset seizures with or without secondary generalisation, in combination with other antiepileptic drugs. Medical uses Brivaracetam is used to treat partial-onset seizures with or without secondary generalisation, in combination with other antiepileptic drugs. Efficacy and tolerability is comparable in general and Intellectual Disability populations. No data are available for its effectiveness and safety in people younger than 16 years of age. Drugs.com Adverse effects The most common adverse effects include sleepiness, dizziness, nausea and vomiting. More rarely, coordination problems and changes in behaviour (such as severe depression, aggression, hostility, impatience, rage, suicidal ideatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |