|
Lipase
In biochemistry, lipase ( ) refers to a class of enzymes that catalyzes the hydrolysis of fats. Some lipases display broad substrate scope including esters of cholesterol, phospholipids, and of lipid-soluble vitamins and sphingomyelinases; however, these are usually treated separately from "conventional" lipases. Unlike esterases, which function in water, lipases "are activated only when adsorbed to an oil–water interface". Lipases perform essential roles in digestion, transport and processing of dietary lipids in most, if not all, organisms. Structure and catalytic mechanism Classically, lipases catalyse the hydrolysis of triglycerides: \begin \text + \ce &\longrightarrow \text + \text \\[4pt] \text + \ce &\longrightarrow \text + \text \\[4pt] \text + \ce &\longrightarrow \text + \text \end Lipases are serine hydrolases, i.e. they function by transesterification generating an acyl serine intermediate. Most lipases act at a specific position on the glycerol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Human Pancreatic Lipase
Pancreatic lipases () are a family of lipolytic enzymes that hydrolyse ester linkages of triglycerides. Lipases are widely distributed in animals, plants and prokaryotes. At least three tissue-specific isozymes exist in higher vertebrates, pancreatic, hepatic and gastric/lingual. These lipases are closely related to each other and to lipoprotein lipase (), which hydrolyses triglycerides of chylomicrons and very low density lipoproteins (VLDL). Pancreatic lipase contains two protein domains. The one toward the N terminus is an α/β hydrolase, whereas the one toward the C terminus plays a role in binding to colipase, a protein needed in order for the lipase to become activated. The most conserved region in all these proteins is centred on a serine residue which has been shown to participate, with a histidine and an aspartic acid residue, in a charge relay system. Such a region is also present in lipases of prokaryotic origin and in lecithin-cholesterol acyltransferase () (L ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serine Hydrolase
Serine hydrolases are one of the largest known enzyme classes comprising approximately ~200 enzymes or 1% of the genes in the human proteome. A defining characteristic of these enzymes is the presence of a particular serine at the active site, which is used for the hydrolysis of substrates. The hydrolysis of the ester or peptide bond proceeds in two steps. First, the acyl part of the substrate (the acid part of an ester or the part of a peptide ending in a carboxyl group) is transferred to the serine, making a new ester or amide bond and releasing the other part of the substrate (the alcohol of an ester or the part of the peptide ending in an amino group) is released. Later, in a slower step, the bond between the serine and the acyl group is hydrolyzed by water or hydroxide ion, regenerating free enzyme. Unlike other, non-catalytic, serines, the reactive serine of these hydrolases is typically activated by a proton relay involving a catalytic triad consisting of the serine, an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cholesterol
Cholesterol is the principal sterol of all higher animals, distributed in body Tissue (biology), tissues, especially the brain and spinal cord, and in Animal fat, animal fats and oils. Cholesterol is biosynthesis, biosynthesized by all animal Cell (biology)#Eukaryotic cells, cells and is an essential structural and cholesterol signaling, signaling component of animal cell membranes. In vertebrates, hepatocyte, hepatic cells typically produce the greatest amounts. In the brain, astrocytes produce cholesterol and transport it to neurons. It is absent among prokaryotes (bacteria and archaea), although there are some exceptions, such as ''Mycoplasma'', which require cholesterol for growth. Cholesterol also serves as a Precursor (chemistry), precursor for the biosynthesis of steroid hormones, bile acid and vitamin D. Elevated levels of cholesterol in the blood, especially when bound to low-density lipoprotein (LDL, often referred to as "bad cholesterol"), may increase the risk of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phospholipid
Phospholipids are a class of lipids whose molecule has a hydrophilic "head" containing a phosphate group and two hydrophobic "tails" derived from fatty acids, joined by an alcohol residue (usually a glycerol molecule). Marine phospholipids typically have omega-3 fatty acids EPA and DHA integrated as part of the phospholipid molecule. The phosphate group can be modified with simple organic molecules such as choline, ethanolamine or serine. Phospholipids are a key component of all cell membranes. They can form lipid bilayers because of their amphiphilic characteristic. In eukaryotes, cell membranes also contain another class of lipid, sterol, interspersed among the phospholipids. The combination provides fluidity in two dimensions combined with mechanical strength against rupture. Purified phospholipids are produced commercially and have found applications in nanotechnology and materials science. The first phospholipid identified in 1847 as such in biological tissues w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Digestion
Digestion is the breakdown of large insoluble food compounds into small water-soluble components so that they can be absorbed into the blood plasma. In certain organisms, these smaller substances are absorbed through the small intestine into the blood stream. Digestion is a form of catabolism that is often divided into two processes based on how food is broken down: mechanical and chemical digestion. The term mechanical digestion refers to the physical breakdown of large pieces of food into smaller pieces which can subsequently be accessed by digestive enzymes. Mechanical digestion takes place in the mouth through Chewing, mastication and in the small intestine through segmentation contractions. In chemical digestion, enzymes break down food into the small compounds that the body can use. In the human digestive system, food enters the mouth and mechanical digestion of the food starts by the action of mastication (chewing), a form of mechanical digestion, and the wetting contact ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different molecules known as product (chemistry), products. Almost all metabolism, metabolic processes in the cell (biology), cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme, pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts include Ribozyme, catalytic RNA molecules, also called ribozymes. They are sometimes descr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha/beta Hydrolase Fold
The alpha/beta hydrolase superfamily is a superfamily of hydrolytic enzymes of widely differing phylogenetic origin and catalytic function that share a common fold. The core of each enzyme is an alpha/beta-sheet (rather than a barrel), containing 8 beta strands connected by 6 alpha helices. The enzymes are believed to have diverged from a common ancestor, retaining little obvious sequence similarity, but preserving the arrangement of the catalytic residues. All have a catalytic triad, the elements of which are borne on loops, which are the best-conserved structural features of the fold. The alpha/beta hydrolase fold includes proteases, lipases, peroxidases, esterases, epoxide hydrolases and dehalogenases. Database The ESTHER database provides a large collection of information about this superfamily of proteins. Subfamilies * 3-oxoadipate enol-lactonase Human proteins containing this domain ABHD10; ABHD11; ABHD12; ABHD12B; ABHD13; ABHD2; ABHD3; ABHD4; A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
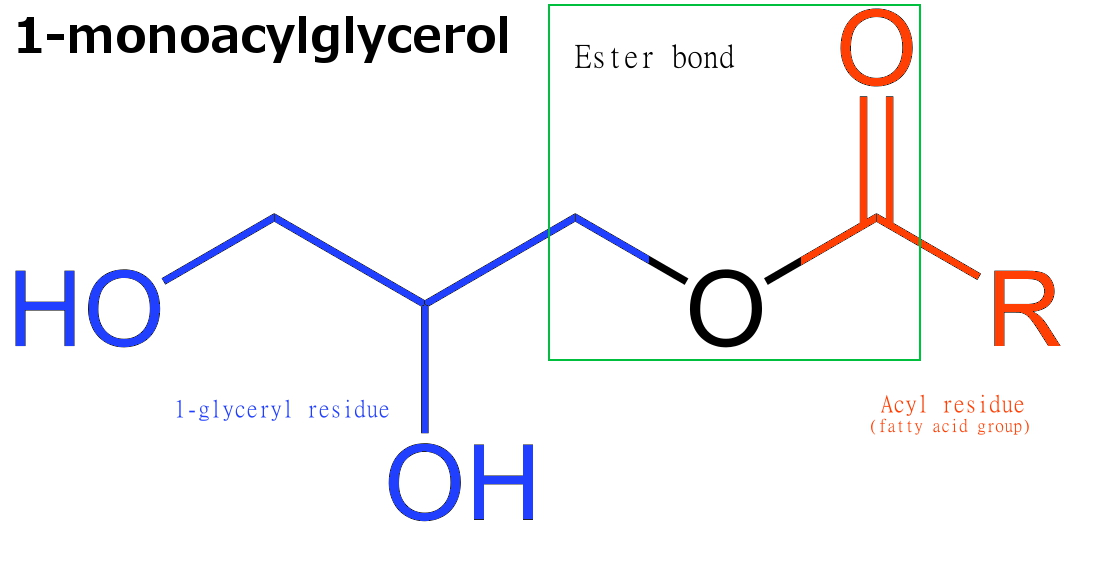
Monoglyceride
Monoglycerides (also: acylglycerols or monoacylglycerols) are a class of glycerides which are composed of a molecule of glycerol linked to a fatty acid via an ester bond. As glycerol contains both primary and secondary alcohol groups two different types of monoglycerides may be formed; 1-monoacylglycerols where the fatty acid is attached to a primary alcohol, or a 2-monoacylglycerols where the fatty acid is attached to the secondary alcohol. Synthesis Monoglycerides are produced both biologically and industrially. They are naturally present at very low levels (0.1-0.2%) in some seed oils such as olive oil, rapeseed oil and cottonseed oil. They are biosynthesized by the enzymatic hydrolysis of triglycerides by lipoprotein lipase and the enzymatic hydrolysis of diglycerides by diacylglycerol lipase; or as an intermediate in the alkanoylation of glycerol to form fats. Several monoglycerides are pharmacologically active (e.g. 2-oleoylglycerol, 2-arachidonoylglycerol). Industrial pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalytic Triad
A catalytic triad is a set of three coordinated amino acid residues that can be found in the active site of some enzymes. Catalytic triads are most commonly found in hydrolase and transferase enzymes (e.g. proteases, amidases, esterases, aminoacylase, acylases, lipases and β-lactamases). An acid-base (chemistry), base-nucleophile triad is a common motif for generating a nucleophilic residue for covalent catalysis. The residues form a charge-relay network to polarise and activate the nucleophile, which attacks the Substrate (chemistry), substrate, forming a covalent intermediate which is then hydrolysed to release the Product (chemistry), product and regenerate free enzyme. The nucleophile is most commonly a serine or cysteine, but occasionally threonine or even selenocysteine. The Protein tertiary structure, 3D structure of the enzyme brings together the triad residues in a precise orientation, even though they may be far apart in the sequence (Protein primary structure, primary ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipid
Lipids are a broad group of organic compounds which include fats, waxes, sterols, fat-soluble vitamins (such as vitamins A, D, E and K), monoglycerides, diglycerides, phospholipids, and others. The functions of lipids include storing energy, signaling, and acting as structural components of cell membranes. Lipids have applications in the cosmetic and food industries, and in nanotechnology. Lipids are broadly defined as hydrophobic or amphiphilic small molecules; the amphiphilic nature of some lipids allows them to form structures such as vesicles, multilamellar/ unilamellar liposomes, or membranes in an aqueous environment. Biological lipids originate entirely or in part from two distinct types of biochemical subunits or "building-blocks": ketoacyl and isoprene groups. Using this approach, lipids may be divided into eight categories: fatty acyls, glycerolipids, glycerophospholipids, sphingolipids, saccharolipids, and polyketides (derived from condensatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sphingomyelinase
Sphingomyelin phosphodiesterase (EC 3.1.4.12, also known as neutral sphingomyelinase, sphingomyelinase, or SMase; systematic name sphingomyelin cholinephosphohydrolase) is a hydrolase enzyme that is involved in sphingolipid metabolism reactions. SMase is a member of the DNase I superfamily of enzymes and is responsible for breaking sphingomyelin (SM) down into phosphocholine and ceramide. The activation of SMase has been suggested as a major route for the production of ceramide in response to cellular stresses. Sphingomyelinase family Five types of SMase have been identified. These are classified according to their cation dependence and pH optima of action and are: * lysosome, Lysosomal Acid sphingomyelinase, acid SMase * Secreted zinc-dependent Acid sphingomyelinase, acid SMase * Magnesium-dependent Neutral sphingomyelinase, neutral SMase * Magnesium-independent Neutral sphingomyelinase, neutral SMase * Alkaline sphingomyelinase, Alkaline SMase Of these, the lysosomal aci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |