|
Lime (material)
Lime is an inorganic material composed primarily of calcium oxides and hydroxides. It is also the name for calcium oxide which occurs as a product of coal-seam fires and in altered limestone xenoliths in volcanic ejecta. The International Mineralogical Association recognizes lime as a mineral with the chemical formula of CaO. The word ''lime'' originates with its earliest use as building mortar and has the sense of ''sticking or adhering''. These materials are still used in large quantities as building and engineering materials (including limestone products, cement, concrete, and mortar), as chemical feedstocks, for sugar refining, and other uses. Lime industries and the use of many of the resulting products date from prehistoric times in both the Old World and the New World. Lime is used extensively for wastewater treatment with ferrous sulfate. The rocks and minerals from which these materials are derived, typically limestone or chalk, are composed primarily of calcium carb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Limestone Quarry
Limestone ( calcium carbonate ) is a type of carbonate sedimentary rock which is the main source of the material lime. It is composed mostly of the minerals calcite and aragonite, which are different crystal forms of . Limestone forms when these minerals precipitate out of water containing dissolved calcium. This can take place through both biological and nonbiological processes, though biological processes, such as the accumulation of corals and shells in the sea, have likely been more important for the last 540 million years. Limestone often contains fossils which provide scientists with information on ancient environments and on the evolution of life. About 20% to 25% of sedimentary rock is carbonate rock, and most of this is limestone. The remaining carbonate rock is mostly dolomite, a closely related rock, which contains a high percentage of the mineral dolomite, . ''Magnesian limestone'' is an obsolete and poorly-defined term used variously for dolomite, for limestone ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lime Kiln
A lime kiln is a kiln used for the calcination of limestone ( calcium carbonate) to produce the form of lime called quicklime ( calcium oxide). The chemical equation for this reaction is : CaCO3 + heat → CaO + CO2 This reaction can take place at anywhere above 840 °C (1544 °F), but is generally considered to occur at 900 °C(1655 °F) (at which temperature the partial pressure of CO2 is 1 atmosphere), but a temperature around 1000 °C (1832 °F) (at which temperature the partial pressure of CO2 is 3.8 atmospheres) is usually used to make the reaction proceed quickly.Parkes, G.D. and Mellor, J.W. (1939). ''Mellor's Modern Inorganic Chemistry'' London: Longmans, Green and Co. Excessive temperature is avoided because it produces unreactive, "dead-burned" lime. Slaked lime ( calcium hydroxide) can be formed by mixing quicklime with water. Early lime use Because it is so readily made by heating limestone, lime must have been known from the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conglomerate (geology)
Conglomerate () is a clastic sedimentary rock that is composed of a substantial fraction of rounded to subangular gravel-size clasts. A conglomerate typically contains a matrix of finer-grained sediments, such as sand, silt, or clay, which fills the interstices between the clasts. The clasts and matrix are typically cemented by calcium carbonate, iron oxide, silica, or hardened clay. Conglomerates form by the consolidation and lithification of gravel. They can be found in sedimentary rock sequences of all ages but probably make up less than 1 percent by weight of all sedimentary rocks. In terms of origin and depositional mechanisms, they are closely related to sandstones and exhibit many of the same types of sedimentary structures, e.g., tabular and trough cross-bedding and graded bedding.Boggs, S. (2006) ''Principles of Sedimentology and Stratigraphy.'', 2nd ed. Prentice Hall, New York. 662 pp. Friedman, G.M. (2003) ''Classification of sediments and sedimentary rocks ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
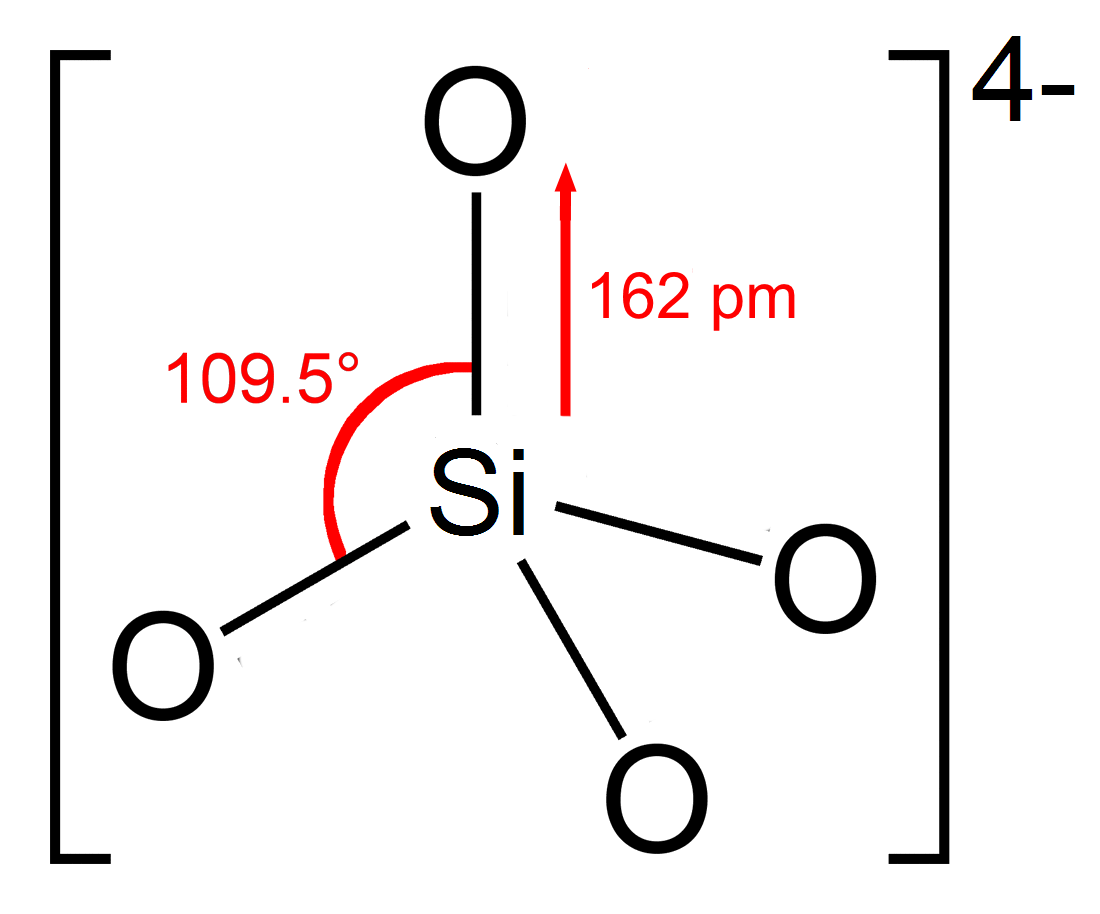
Silicate
In chemistry, a silicate is any member of a family of polyatomic anions consisting of silicon and oxygen, usually with the general formula , where . The family includes orthosilicate (), metasilicate (), and pyrosilicate (, ). The name is also used for any salt of such anions, such as sodium metasilicate; or any ester containing the corresponding chemical group, such as tetramethyl orthosilicate. The name "silicate" is sometimes extended to any anions containing silicon, even if they do not fit the general formula or contain other atoms besides oxygen; such as hexafluorosilicate .Most commonly, silicates are encountered as silicate minerals. For diverse manufacturing, technological, and artistic needs, silicates are versatile materials, both natural (such as granite, gravel, and garnet) and artificial (such as Portland cement, ceramics, glass, and waterglass). Structural principles In all silicates, silicon atom occupies the center of an idealized tetrahedro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Argillaceous Minerals
Clay minerals are hydrous aluminium phyllosilicates (e.g. kaolin, Al2 Si2 O5( OH)4), sometimes with variable amounts of iron, magnesium, alkali metals, alkaline earths, and other cations found on or near some planetary surfaces. Clay minerals form in the presence of water and have been important to life, and many theories of abiogenesis involve them. They are important constituents of soils, and have been useful to humans since ancient times in agriculture and manufacturing. Properties Clay is a very fine-grained geologic material that develops plasticity when wet, but becomes hard, brittle and non–plastic upon drying or firing. It is a very common material, and is the oldest known ceramic. Prehistoric humans discovered the useful properties of clay and used it for making pottery. The chemistry of clay, including its capacity to retain nutrient cations such as potassium and ammonium, is important to soil fertility. Because the individual particles in clay are less th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Marl
Marl is an earthy material rich in carbonate minerals, clays, and silt. When hardened into rock, this becomes marlstone. It is formed in marine or freshwater environments, often through the activities of algae. Marl makes up the lower part of the cliffs of Dover, and the Channel Tunnel follows these marl layers between France and the United Kingdom. Marl is also a common sediment in post-glacial lakes, such as the marl ponds of the northeastern United States. Marl has been used as a soil conditioner and neutralizing agent for acid soil and in the manufacture of cement. Description Marl or marlstone is a carbonate-rich mud or mudstone which contains variable amounts of clays and silt. The term was originally loosely applied to a variety of materials, most of which occur as loose, earthy deposits consisting chiefly of an intimate mixture of clay and calcium carbonate, formed under freshwater conditions. These typically contain 35–65% clay and 65–35% carbonate. The te ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oolite
Oolite or oölite (''egg stone'') is a sedimentary rock formed from ooids, spherical grains composed of concentric layers. The name derives from the Ancient Greek word for egg (ᾠόν). Strictly, oolites consist of ooids of diameter 0.25–2 millimetres; rocks composed of ooids larger than 2 mm are called pisolites. The term ''oolith'' can refer to oolite or individual ooids. Composition Ooids are most commonly composed of calcium carbonate (calcite or aragonite), but can be composed of phosphate, clays, chert, dolomite or iron minerals, including hematite. Dolomitic and chert ooids are most likely the result of the replacement of the original texture in limestone. Oolitic hematite occurs at Red Mountain near Birmingham, Alabama, along with oolitic limestone. They are usually formed in warm, supersaturated, shallow, highly agitated marine water intertidal environments, though some are formed in inland lakes. The mechanism of formation starts with a small fra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Marble
Marble is a metamorphic rock composed of recrystallized carbonate minerals, most commonly calcite or dolomite. Marble is typically not foliated (layered), although there are exceptions. In geology, the term ''marble'' refers to metamorphosed limestone, but its use in stonemasonry more broadly encompasses unmetamorphosed limestone. Marble is commonly used for sculpture and as a building material. Etymology The word "marble" derives from the Ancient Greek (), from (), "crystalline rock, shining stone", perhaps from the verb (), "to flash, sparkle, gleam"; R. S. P. Beekes has suggested that a " Pre-Greek origin is probable". This stem is also the ancestor of the English word "marmoreal," meaning "marble-like." While the English term "marble" resembles the French , most other European languages (with words like "marmoreal") more closely resemble the original Ancient Greek. Physical origins Marble is a rock resulting from metamorphism of sedimentary carbonate ro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
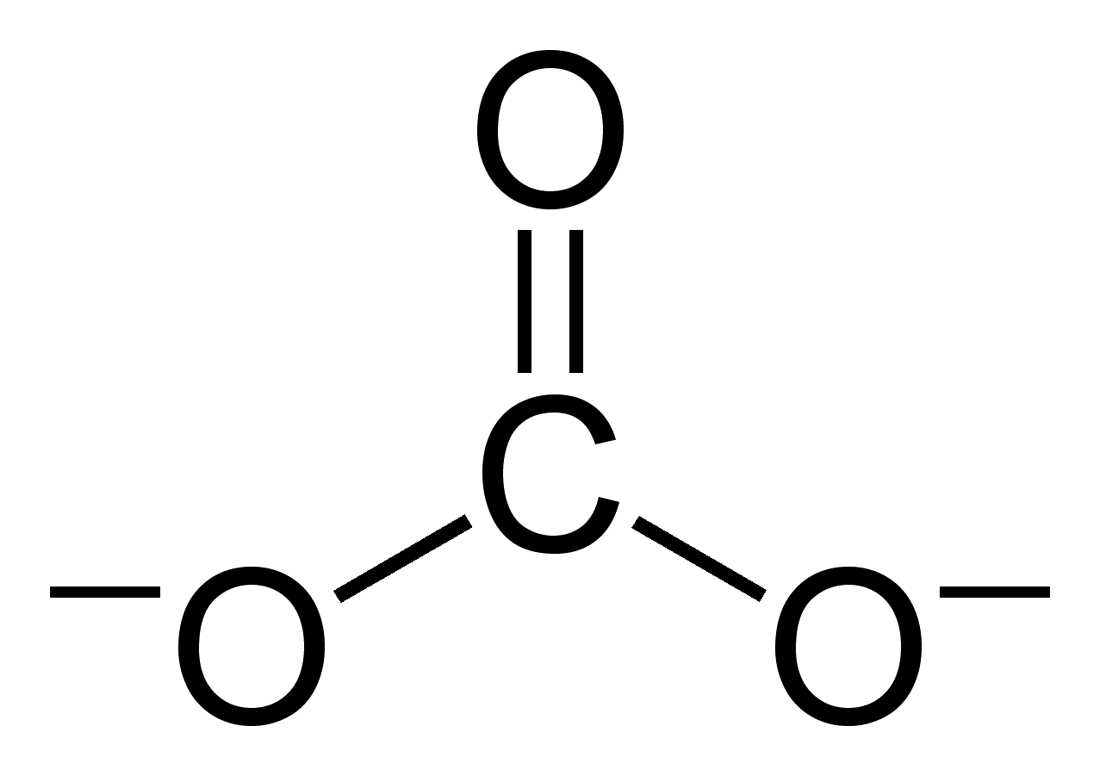
Carbonate
A carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word ''carbonate'' may also refer to a carbonate ester, an organic compound containing the carbonate group C(=O)(O–)2. The term is also used as a verb, to describe carbonation: the process of raising the concentrations of carbonate and bicarbonate ions in water to produce carbonated water and other carbonated beverageseither by the addition of carbon dioxide gas under pressure or by dissolving carbonate or bicarbonate salts into the water. In geology and mineralogy, the term "carbonate" can refer both to carbonate minerals and carbonate rock (which is made of chiefly carbonate minerals), and both are dominated by the carbonate ion, . Carbonate minerals are extremely varied and ubiquitous in chemically precipitated sedimentary rock. The most common are calcite or calcium carbonate, CaCO3, the chief constituent of limestone (as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quicklime
Calcium oxide (CaO), commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline, crystalline solid at room temperature. The broadly used term "'' lime''" connotes calcium-containing inorganic materials, in which carbonates, oxides and hydroxides of calcium, silicon, magnesium, aluminium, and iron predominate. By contrast, ''quicklime'' specifically applies to the single chemical compound calcium oxide. Calcium oxide that survives processing without reacting in building products such as cement is called free lime. Quicklime is relatively inexpensive. Both it and a chemical derivative ( calcium hydroxide, of which quicklime is the base anhydride) are important commodity chemicals. Preparation Calcium oxide is usually made by the thermal decomposition of materials, such as limestone or seashells, that contain calcium carbonate (CaCO3; mineral calcite) in a lime kiln. This is accomplished by heating the material to above ,Merc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Slaked Lime
Calcium hydroxide (traditionally called slaked lime) is an inorganic compound with the chemical formula Ca( OH)2. It is a colorless crystal or white powder and is produced when quicklime (calcium oxide) is mixed or slaked with water. It has many names including hydrated lime, caustic lime, builders' lime, slaked lime, cal, and pickling lime. Calcium hydroxide is used in many applications, including food preparation, where it has been identified as E number E526. Limewater, also called milk of lime, is the common name for a saturated solution of calcium hydroxide. Properties Calcium hydroxide is poorly soluble in water, with a retrograde solubility increasing from 0.66 g/L at 100 °C to 1.89 g/L at 0 °C. With a solubility product ''K''sp of 5.02 at 25 °C, its dissociation in water is large enough that its solutions are basic according to the following dissolution reaction: : Ca(OH)2 → Ca2+ + 2 OH− At ambient temperature, calcium hydroxide ( portlandite) di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Agricultural Lime
Agricultural lime, also called aglime, agricultural limestone, garden lime or liming, is a soil additive made from pulverized limestone or chalk. The primary active component is calcium carbonate. Additional chemicals vary depending on the mineral source and may include calcium oxide. Unlike the types of lime called quicklime (calcium oxide) and slaked lime (calcium hydroxide), powdered limestone does not require lime burning in a lime kiln; it only requires milling. All of these types of lime are sometimes used as soil conditioners, with a common theme of providing a base to correct acidity, but lime for farm fields today is often crushed limestone. Historically, liming of farm fields in centuries past was often done with burnt lime; the difference is at least partially explained by the fact that affordable mass-production-scale fine milling of stone and ore relies on technologies developed since the mid-19th century. Some effects of agricultural lime on soil are: * it in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |