|
Levoketoconazole
Levoketoconazole, sold under the brand name Recorlev, is a steroidogenesis inhibitor that is used for the treatment of Cushing's syndrome. Levoketoconazole was approved for medical use in the United States in December 2021. Levoketoconazole is the levorotatory or (2''S'',4''R'') enantiomer of ketoconazole, and it is an inhibitor of the enzymes CYP11B1 (11β-hydroxylase), CYP17A1 (17α-hydroxylase/17,20-lyase), and CYP21A2 (21-hydroxylase). It inhibits glucocorticoid biosynthesis and hence circulating levels of glucocorticoids, thereby treating Cushing's syndrome. In addition to its increased potency, the drug is 12-fold less potent than racemic ketoconazole in inhibiting CYP7A1 (cholesterol 7α-hydroxylase), theoretically resulting in further reduced interference with bile acid production and metabolite elimination and therefore less risk of hepatotoxicity. Levoketoconazole has also been found to inhibit CYP11A1 (cholesterol side-chain cleavage enzyme) and CYP51A1 Lanostero ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steroidogenesis Inhibitor
A steroidogenesis inhibitor, also known as a steroid biosynthesis inhibitor, is a type of drug which inhibits one or more of the enzymes that are involved in the process of steroidogenesis, the biosynthesis of endogenous steroids and steroid hormones. They may inhibit the production of cholesterol and other sterols, sex steroids such as androgens, estrogens, and progestogens, corticosteroids such as glucocorticoids and mineralocorticoids, and neurosteroids. They are used in the treatment of a variety of medical conditions that depend on endogenous steroids. Steroidogenesis inhibitors are analogous in effect and use to antigonadotropins (which specifically inhibit gonadal sex steroid production), but work via a different mechanism of action; whereas antigonadotropins suppress gonadal production of sex steroids by effecting negative feedback on and thereby suppressing the hypothalamic–pituitary–gonadal axis, steroidogenesis inhibitors directly inhibit the enzymatic biosy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketoconazole
Ketoconazole, sold under the brand name Nizoral, among others, is an antiandrogen, antifungal drug, antifungal, and antiglucocorticoid medication used to treat a number of fungal infections. Applied to the skin it is used for fungal skin infections such as tinea, cutaneous candidiasis, pityriasis versicolor, dandruff, and seborrhoeic dermatitis, seborrheic dermatitis. Taken oral administration, by mouth it is a less preferred option and recommended for only severe infections when other agents cannot be used. Other uses include treatment of hirsutism, excessive male-patterned hair growth in women and Cushing's syndrome. Common side effects when transdermal administration, applied to the skin include redness. Common side effects when taken by mouth include nausea, headache, and liver problems. Liver problems may result in death or the need for a liver transplantation. Other severe side effects when taken orally include QT prolongation, adrenocortical insufficiency, and anaphyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oral Administration
Oral administration is a route of administration whereby a substance is taken through the Human mouth, mouth, swallowed, and then processed via the digestive system. This is a common route of administration for many medications. Oral administration can be easier and less painful than other routes of administration, such as Injection (medicine), injection. However, the onset of action is relatively low, and the effectiveness is reduced if it is not absorbed properly in the digestive system, or if it is broken down by digestive enzymes before it can reach the bloodstream. Some medications may cause gastrointestinal side effects, such as nausea or vomiting, when taken orally. Oral administration can also only be applied to conscious patients, and patients able to swallow. Terminology ''Per os'' (; ''P.O.'') is an adverbial phrase meaning literally from Latin "through the mouth" or "by mouth". The expression is used in medicine to describe a treatment that is taken orally (but not ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enantiopure Drugs
An enantiopure drug is a pharmaceutical available in one specific enantiomeric form. Most biomolecules (proteins, sugars, etc.) are present in only one of many chiral forms, so different enantiomers of a chiral drug molecule bind differently (or not at all) to target receptors. The use of a drug with a single enantiomer intends to make it more effective. One enantiomer of a drug may have a desired beneficial effect while the other may cause serious and undesired side effects, or sometimes even beneficial but entirely different effects. The desired enantiomer is known as an ''eutomer'' while the undesired enantiomer is known as the ''distomer''. When equal amounts of both enantiomers are found in a mixture, the mixture is known as a racemic mixture. If a mixture for a drug does not have a 1:1 ratio of its enantiomers it is a candidate for an enantiopure drug. Advances in industrial chemical processes have made it economical for pharmaceutical manufacturers to take drugs that wer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
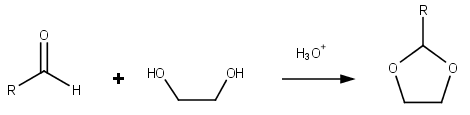
Dioxolanes
Dioxolane is a heterocyclic acetal with the chemical formula (CH2)2O2CH2. It is related to tetrahydrofuran (THF) by replacement of the methylene group (CH2) at the 2-position with an oxygen atom. The corresponding saturated 6-membered C4O2 rings are called dioxanes. The isomeric 1,2-dioxolane (wherein the two oxygen centers are adjacent) is a peroxide. 1,3-dioxolane is used as a solvent and as a comonomer in polyacetals. As a class of compounds Dioxolanes are a group of organic compounds containing the dioxolane ring. Dioxolanes can be prepared by acetalization of aldehydes and ketalization of ketones with ethylene glycol. (+)-''cis''-Dioxolane is the trivial name for which is a muscarinic acetylcholine receptor agonist. Protecting groups Organic compounds containing carbonyl groups sometimes need protection so that they do not undergo reactions during transformations of other functional groups that may be present. A variety of approaches to protection and deprotection ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CYP17A1 Inhibitors
A CYP17A1 inhibitor is a type of drug that enzyme inhibitor, inhibits the enzyme CYP17A1. CYP17A1 inhibitors work by blocking specific enzyme functions, impacting androgen biosynthesis. Mechanism of action CYP17A1 inhibitors may inhibit one or both of the enzyme’s functions: 17α-hydroxylase and 17,20-lyase. Some inhibitors are selective and target only the 17,20-lyase function, while others inhibit both functions. By inhibiting these enzymatic functions, CYP17A1 inhibitors prevent the conversion of pregnane steroids into androgens like testosterone. This action classifies them as androgen biosynthesis inhibitors and functional antiandrogens. Examples Examples of CYP17A1 inhibitors include the older drug ketoconazole and the newer drugs abiraterone acetate, orteronel, galeterone, and seviteronel. Clinical uses CYP17A1 inhibitors, such as abiraterone acetate, are primarily used in the treatment of prostate cancer. These drugs reduce androgen levels, which helps to slow ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CYP3A4 Inhibitors
Cytochrome P450 3A4 (abbreviated CYP3A4) () is an important enzyme in the body, mainly found in the liver and in the intestine, which in humans is encoded by ''CYP3A4'' gene. It oxidizes small foreign organic molecules (xenobiotics), such as toxins or drugs, so that they can be removed from the body. It is highly homologous to CYP3A5, another important CYP3A enzyme. While many drugs are deactivated by CYP3A4, there are also some drugs that are ''activated'' by the enzyme. Some substances, such as some drugs and furanocoumarins present in grapefruit juice, interfere with the action of CYP3A4. These substances will, therefore, either amplify or weaken the action of those drugs that are modified by CYP3A4. CYP3A4 is a member of the cytochrome P450 family of oxidizing enzymes. Several other members of this family are also involved in drug metabolism, but CYP3A4 is the most common and the most versatile one. Like all members of this family, it is a hemoprotein, i.e. a protein cont ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cholesterol Side-chain Cleavage Enzyme Inhibitors
Cholesterol side-chain cleavage enzyme is commonly referred to as P450scc, where "scc" is an abbreviation for side-chain cleavage. P450scc is a mitochondrial enzyme that catalyzes conversion of cholesterol to pregnenolone. This is the first reaction in the process of steroidogenesis in all mammalian tissues that specialize in the production of various steroid hormones. P450scc is a member of the cytochrome P450 superfamily of enzymes (family 11, subfamily A, polypeptide 1) and is encoded by the gene. Nomenclature The systematic name of this enzyme class is cholesterol, reduced-adrenal-ferredoxin:oxygen oxidoreductase (side-chain-cleaving). Other names include: * C27-side-chain cleavage enzyme * cholesterol 20-22-desmolase * cholesterol C20-22 desmolase * cholesterol desmolase * cholesterol side-chain cleavage enzyme * cholesterol side-chain-cleaving enzyme * cytochrome P-450scc * desmolase, steroid 20-22 * enzymes, cholesterol side-chain-cleaving * steroid 20-22 desm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloroarenes
In organic chemistry, an aryl halide (also known as a haloarene) is an aromatic compound in which one or more hydrogen atoms directly bonded to an aromatic ring are replaced by a halide ion (such as fluorine F''−'', chlorine Cl−1,−3,−5, bromine Br−1, or iodine I−). Aryl halides are distinct from haloalkanes (alkyl halides) due to significant differences in their methods of preparation, chemical reactivity, and physical properties. The most common and important members of this class are aryl chlorides, but the group encompasses a wide range of derivatives with diverse applications in organic synthesis, pharmaceuticals, and materials science. Classification according to halide Aryl fluorides Aryl fluorides are used as synthetic intermediates, e.g. for the preparation of pharmaceuticals, pesticides, and liquid crystals. The conversion of diazonium salts is a well established route to aryl fluorides. Thus, anilines are precursors to aryl fluorides. In the classic Schiemann ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetamides
Acetamide (systematic name: ethanamide) is an organic compound with the formula CH3CONH2. It is an amide derived from ammonia and acetic acid. It finds some use as a plasticizer and as an industrial solvent. The related compound ''N'',''N''-dimethylacetamide (DMA) is more widely used, but it is not prepared from acetamide. Acetamide can be considered an intermediate between acetone, which has two methyl (CH3) groups either side of the carbonyl (CO), and urea which has two amide (NH2) groups in those locations. Acetamide is also a naturally occurring mineral with the IMA symbol: Ace. Production Laboratory scale Acetamide can be produced in the laboratory from ammonium acetate by dehydration: : H4CH3CO2] → CH3C(O)NH2 + H2O Alternatively acetamide can be obtained in excellent yield via ammonolysis of acetylacetone under conditions commonly used in reductive amination. It can also be made from anhydrous acetic acid, acetonitrile and very well dried hydrogen chloride gas, u ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |