|
Lead(IV) Sulfide
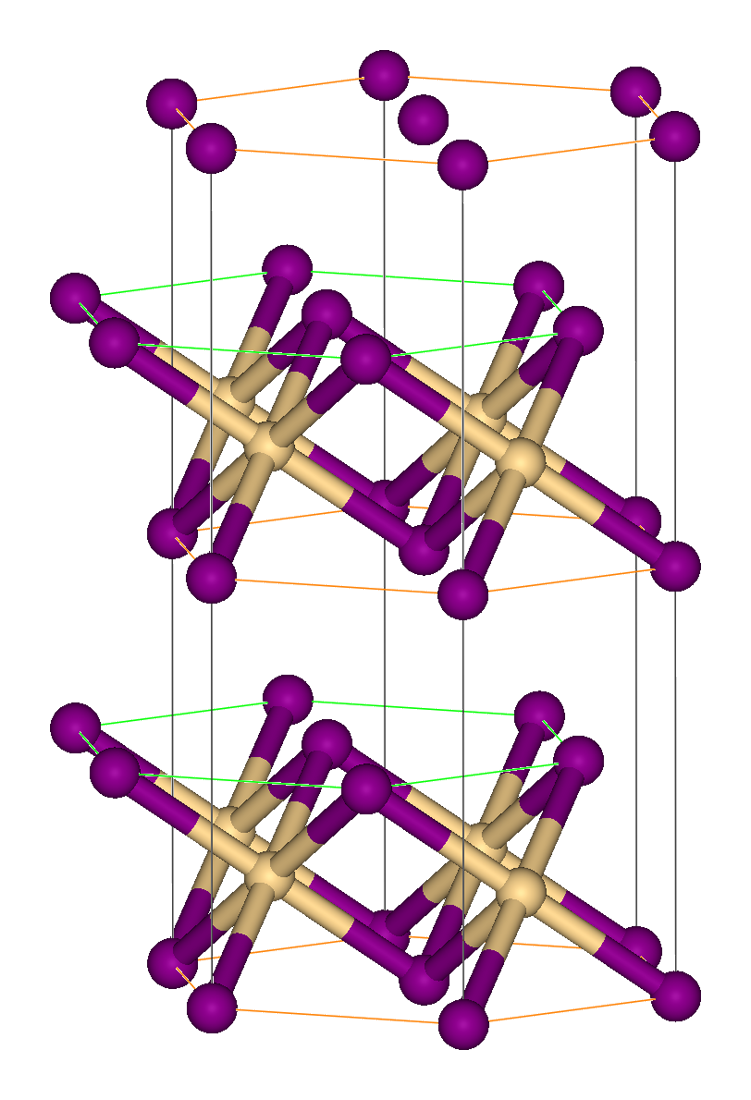
Lead(IV) sulfide is a chemical compound with the formula Pb S2. This material is generated by the reaction of the more common lead(II) sulfide, PbS, with sulfur at >600 °C and at high pressures. PbS2, like the related tin(IV) sulfide SnS2, crystallises in the cadmium iodide motif, which indicates that Pb should be assigned the formal oxidation state In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ... of 4+. Lead(IV) sulfide is a p-type semiconductor, and is also a thermoelectric material. References Lead(IV) compounds Disulfides {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Chemistry (journal)
''Inorganic Chemistry'' is a biweekly peer-reviewed scientific journal published by the American Chemical Society since 1962. It covers research in all areas of inorganic chemistry. The current editor-in-chief is Stefanie Dehnen ( Karlsruhe Institute of Technology). Abstracting and indexing The journal is abstracted and indexed in: According to the ''Journal Citation Reports'', the journal has a 2022 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a type of journal ranking. Journals with higher impact factor values are considered more prestigious or important within their field. The Impact Factor of a journa ... of 4.6. See also * '' Organometallics'' References External links * American Chemical Society academic journals Biweekly journals Academic journals established in 1962 English-language journals Inorganic chemistry journals {{chem-journal-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rhombohedral
In geometry, a rhombohedron (also called a rhombic hexahedron or, inaccurately, a rhomboid) is a special case of a parallelepiped in which all six faces are congruent rhombus, rhombi. It can be used to define the rhombohedral lattice system, a Honeycomb (geometry), honeycomb with rhombohedral cells. A rhombohedron has two opposite Apex_(geometry), apices at which all face angles are equal; a prolate rhombohedron has this common angle acute, and an oblate rhombohedron has an obtuse angle at these vertices. A cube is a special case of a rhombohedron with all sides square. Special cases The common angle at the two apices is here given as \theta. There are two general forms of the rhombohedron: oblate (flattened) and prolate (stretched). In the oblate case \theta > 90^\circ and in the prolate case \theta < 90^\circ. For the figure is a cube. Certain proportions of the rhombs give rise to some well-known special cases. These typically occur in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pearson Symbol
The Pearson symbol, or Pearson notation, is used in crystallography as a means of describing a crystal structure. It was originated by William Burton Pearson and is used extensively in Pearson's handbook of crystallographic data for intermetallic phases. The symbol is made up of two letters followed by a number. For example: * Diamond structure, cF8 * Rutile structure, tP6 Construction The two letters in the Pearson symbol specify the Bravais lattice, and more specifically, the lower-case letter specifies the Crystal system, crystal family, while the upper-case letter the Lattice (group), lattice type. The number at the end of the Pearson symbol gives the number of the atoms in the conventional unit cell (atoms which satisfy 1 > x,y,z \geq 0 for the atom's position (x,y,z) in the unit cell). [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken or new bonds formed or both. There are four major types of compounds, distinguished by how the constituent atoms are bonded together. Molecular compounds are held together by covalent bonds; ionic compounds are held together by ionic bonds; intermetallic compounds are held together by metallic bonds; coordination complexes are held together by coordinate covalent bonds. Non-stoichiometric compounds form a disputed marginal case. A chemical formula specifies the number of atoms of each element in a compound molecule, usin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lead
Lead () is a chemical element; it has Chemical symbol, symbol Pb (from Latin ) and atomic number 82. It is a Heavy metal (elements), heavy metal that is density, denser than most common materials. Lead is Mohs scale, soft and Ductility, malleable, and also has a relatively low melting point. When freshly cut, lead is a shiny gray with a hint of blue. It tarnishes to a dull gray color when exposed to air. Lead has the highest atomic number of any stable nuclide, stable element and three of its isotopes are endpoints of major nuclear decay chains of heavier elements. Lead is a relatively unreactive post-transition metal. Its weak metallic character is illustrated by its Amphoterism, amphoteric nature; lead and lead oxides react with acids and base (chemistry), bases, and it tends to form covalent bonds. Lead compounds, Compounds of lead are usually found in the +2 oxidation state rather than the +4 state common with lighter members of the carbon group. Exceptions are mostly limited ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with the chemical formula S8. Elemental sulfur is a bright yellow, crystalline solid at room temperature. Sulfur is the tenth most abundant element by mass in the universe and the fifth most common on Earth. Though sometimes found in pure, native form, sulfur on Earth usually occurs as sulfide and sulfate minerals. Being abundant in native form, sulfur was known in ancient times, being mentioned for its uses in ancient India, ancient Greece, China, and ancient Egypt. Historically and in literature sulfur is also called brimstone, which means "burning stone". Almost all elemental sulfur is produced as a byproduct of removing sulfur-containing contaminants from natural gas and petroleum.. Downloahere Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lead(II) Sulfide
Lead(II) sulfide (also spelled '' sulphide'') is an inorganic compound with the formula Pb S. Galena is the principal ore and the most important compound of lead. It is a semiconducting material with niche uses. Formation, basic properties, related materials Addition of hydrogen sulfide or sulfide salts to a solution containing a lead salt, such as PbCl2, gives a black precipitate of lead sulfide. : Pb2+ + H2S → PbS↓ + 2 H+ This reaction is used in qualitative inorganic analysis. The presence of hydrogen sulfide or sulfide ions may be tested using "lead acetate paper." Like the related materials PbSe and PbTe, PbS is a semiconductor. In fact, lead sulfide was one of the earliest materials to be used as a semiconductor. Lead sulfide crystallizes in the sodium chloride motif, unlike many other IV-VI semiconductors. Since PbS is the main ore of lead, much effort has focused on its conversion. A major process involves smelting of PbS followed by reduction of the re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with the chemical formula S8. Elemental sulfur is a bright yellow, crystalline solid at room temperature. Sulfur is the tenth most abundant element by mass in the universe and the fifth most common on Earth. Though sometimes found in pure, native form, sulfur on Earth usually occurs as sulfide and sulfate minerals. Being abundant in native form, sulfur was known in ancient times, being mentioned for its uses in ancient India, ancient Greece, China, and ancient Egypt. Historically and in literature sulfur is also called brimstone, which means "burning stone". Almost all elemental sulfur is produced as a byproduct of removing sulfur-containing contaminants from natural gas and petroleum.. Downloahere Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tin(IV) Sulfide
Tin(IV) sulfide is a compound with the chemical formula, formula . A brown, water-insoluble solid, it is a semiconductor with band gap 2.2 eV. It occurs naturally as the rare mineral berndtite. Synthesis and structure :file:SnS2-bas.png, left, Fragment of the SnS2 lattice. Color code: yellow = S, gray = Sn., 120px The compound precipitates as a brown solid upon the addition of to solutions containing tin(IV) species. This reaction is reversed at low pH. It can also be prepared by heating finely ground Sn with excess sulfur. The compound crystallizes in the cadmium iodide motif, with the Sn(IV) situated in "octahedral holes' defined by six sulfide centers. The material reacts with sulfide salts to give a series of thiostannates with the formula . A simplified equation for this depolymerization reaction is: : + → Potential uses Crystalline has a bronze color and is used in decorative coating where it is known as mosaic gold. Tin (IV) sulfide has various uses in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cadmium Iodide
Cadmium iodide is an inorganic compound with the formula CdI2. It is a white hygroscopic solid. It also can be obtained as a mono- and tetrahydrate. It has few applications. It is notable for its crystal structure, which is typical for compounds of the form MX2 with strong polarization effects. Preparation Cadmium iodide is prepared by the addition of cadmium metal, or its oxide, hydroxide or carbonate to hydroiodic acid. Also, the compound can be made by heating cadmium with iodine. Applications Historically, cadmium iodide was used as a catalyst for the Henkel process, a high-temperature isomerisation of dipotassium phthalate to yield the terephthalate. The salt was then treated with acetic acid to yield potassium acetate and commercially valuable terephthalic acid. While uneconomical compared to the production of terephthalic acid from ''p''-xylene, the Henkel method has been proposed as a potential route to produce terephthalic acid from furfural. As existing B ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidation State
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. Conceptually, the oxidation state may be positive, negative or zero. Beside nearly-pure ionic bonding, many covalent bonds exhibit a strong ionicity, making oxidation state a useful predictor of charge. The oxidation state of an atom does not represent the "real" charge on that atom, or any other actual atomic property. This is particularly true of high oxidation states, where the ionization energy required to produce a multiply positive ion is far greater than the energies available in chemical reactions. Additionally, the oxidation states of atoms in a given compound may vary depending on Electronegativities of the elements (data page), the choice of electronegativity scale used in their calculation. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lead(IV) Compounds
Lead () is a chemical element; it has symbol Pb (from Latin ) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cut, lead is a shiny gray with a hint of blue. It tarnishes to a dull gray color when exposed to air. Lead has the highest atomic number of any stable element and three of its isotopes are endpoints of major nuclear decay chains of heavier elements. Lead is a relatively unreactive post-transition metal. Its weak metallic character is illustrated by its amphoteric nature; lead and lead oxides react with acids and bases, and it tends to form covalent bonds. Compounds of lead are usually found in the +2 oxidation state rather than the +4 state common with lighter members of the carbon group. Exceptions are mostly limited to organolead compounds. Like the lighter members of the group, lead tends to bond with itself; it can form chains and polyhedra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |