|
Lavoisier
Antoine-Laurent de Lavoisier ( ; ; 26 August 17438 May 1794), CNRS () also Antoine Lavoisier after the French Revolution, was a French nobleman and who was central to the 18th-century |
Marie-Anne Paulze Lavoisier
Marie-Anne Pierrette Paulze Lavoisier, later Imperial Count, Countess von Rumford, (20 January 1758 in Montbrison, Loire, France – 10 February 1836) was a French chemist and noblewoman. Madame Lavoisier's first husband was the chemist and nobleman Antoine Lavoisier. She acted as his laboratory companion, using her linguistic skills to write up his work and bring it to an international audience. She also played a pivotal role in the translation of several scientific works, and was instrumental to the standardization of the scientific method. Biography Her father, Jacques Paulze, worked primarily as a parliamentary lawyer and financier. Most of his income came from running the Ferme Générale (the General Farm) which was a private consortium of financiers who paid the French monarchy for the privilege of collecting certain taxes. Her mother, Claudine Thoynet Paulze, died in 1761, leaving behind Marie-Anne, then aged 3, and two sons. After her mother's death Paulze was placed ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
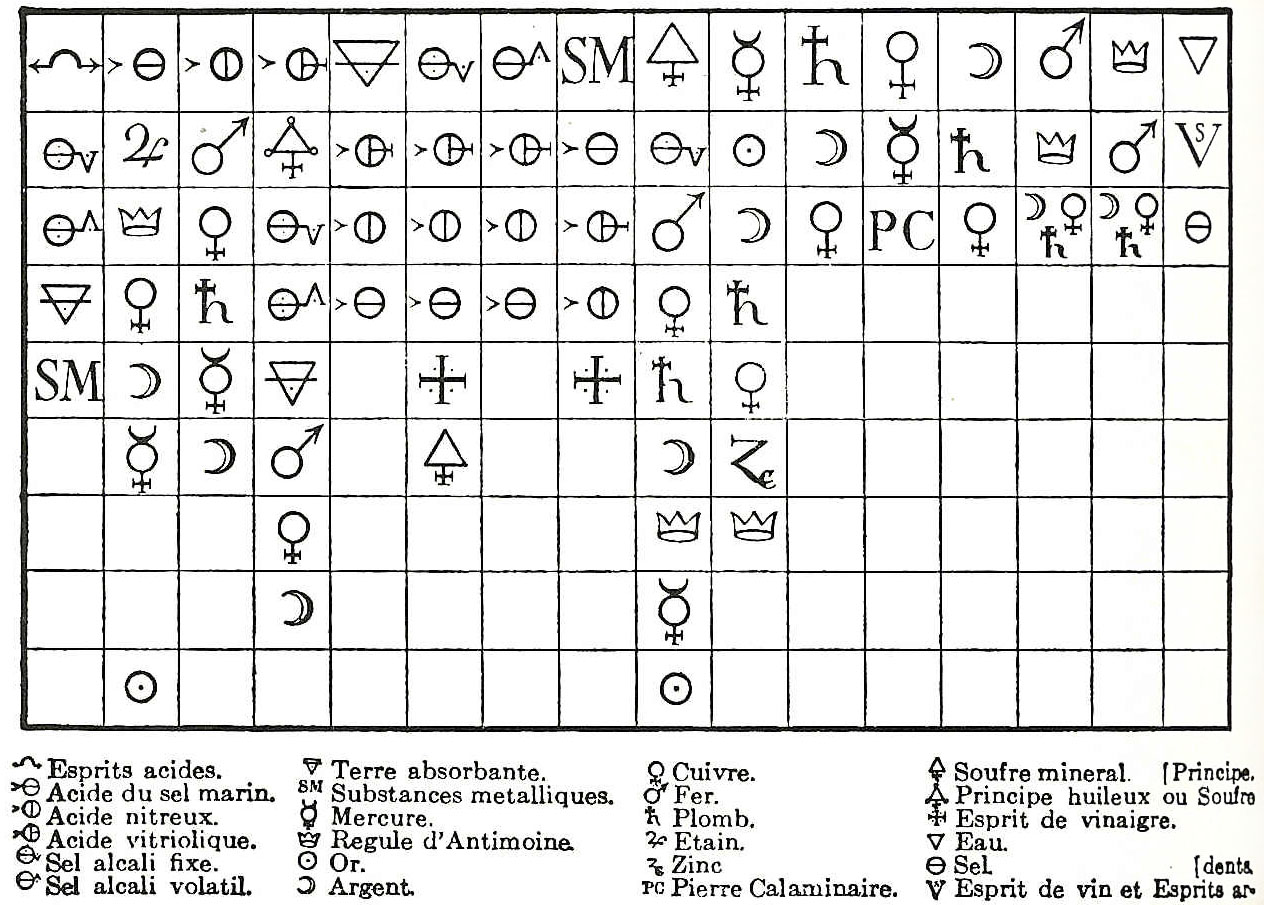
History Of Chemistry
The history of chemistry represents a time span from ancient history to the present. By 1000 BC, civilizations used technologies that would eventually form the basis of the various branches of chemistry. Examples include the discovery of fire, extracting metals from ores, making pottery and glazes, fermenting beer and wine, extracting chemicals from plants for medicine and perfume, rendering fat into soap, making glass, and making alloys like bronze. The protoscience of chemistry, and alchemy, was unsuccessful in explaining the nature of matter and its transformations. However, by performing experiments and recording the results, alchemists set the stage for modern chemistry. The history of chemistry is intertwined with the history of thermodynamics, especially through the work of Willard Gibbs. Ancient history Early humans Fire Arguably the first chemical reaction used in a controlled manner was combustion, fire. However, for millennia fire was seen simply as a mystical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Revolution
In the history of chemistry, the chemical revolution, also called the ''first chemical revolution'', was the reformulation of chemistry during the seventeenth and eighteenth centuries, which culminated in the law of conservation of mass and the oxygen theory of combustion. During the 19th and 20th century, this transformation was credited to the work of the French chemist Antoine Lavoisier (the " father of modern chemistry"). However, recent work on the history of early modern chemistry considers the chemical revolution to consist of gradual changes in chemical theory and practice that emerged over a period of two centuries. The so-called Scientific Revolution took place during the sixteenth and seventeenth centuries whereas the chemical revolution took place during the seventeenth and eighteenth centuries. Primary factors Several factors led to the first chemical revolution. First, there were the forms of gravimetric analysis that emerged from alchemy and new kinds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Portrait Of Antoine-Laurent Lavoisier And His Wife
The ''Portrait of Antoine and Marie-Anne Lavoisier'' () is a double portrait of the French chemist Antoine Lavoisier and his wife and collaborator Marie-Anne Pierrette Paulze, commissioned from the French painter Jacques-Louis David in 1788 by Marie-Anne (who had been taught drawing by David). Description The work is painted in Oil painting, oils on a canvas of 259.7 × 194.6 cm. It shows the couple in Lavoisier's office, with a wood-paneled floor and walls of false marble with three classical pilasters. In the center, the couple face the viewer with both their heads in three-quarters profile. Marie-Anne is shown standing, looking at the viewer. Her costume is that in fashion at the end of the 18th century – powdered hair, a white dress with a lace-edged ruffled neckline, and a blue fabric sash. She rests on her husband's shoulder, with her right hand leaning on the table. Antoine Lavoisier is seated, wearing a black vest, culottes, stockings and buckled shoes, a wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), nonmetal, and a potent oxidizing agent that readily forms oxides with most elements as well as with other chemical compound, compounds. Oxygen is abundance of elements in Earth's crust, the most abundant element in Earth's crust, making up almost half of the Earth's crust in the form of various oxides such as water, carbon dioxide, iron oxides and silicates.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. It is abundance of chemical elements, the third-most abundant element in the universe after hydrogen and helium. At standard temperature and pressure, two oxygen atoms will chemical bond, bind covalent bond, covalently to form dioxygen, a colorless and odorless diatomic gas with the chemical formula ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Law Of Conservation Of Mass
In physics and chemistry, the law of conservation of mass or principle of mass conservation states that for any system closed to all transfers of matter the mass of the system must remain constant over time. The law implies that mass can neither be created nor destroyed, although it may be rearranged in space, or the entities associated with it may be changed in form. For example, in chemical reactions, the mass of the chemical components before the reaction is equal to the mass of the components after the reaction. Thus, during any chemical reaction and low-energy thermodynamic processes in an isolated system, the total mass of the reactants, or starting materials, must be equal to the mass of the products. The concept of mass conservation is widely used in many fields such as chemistry, mechanics, and fluid dynamics. Historically, mass conservation in chemical reactions was primarily demonstrated in the 17th century and finally confirmed by Antoine Lavoisier in the late 18th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Éleuthère Irénée Du Pont
Éleuthère Irénée du Pont de Nemours ( , ; 24 June 1771 – 31 October 1834) was a French-American chemist and industrialist who founded the gunpowder manufacturer E. I. du Pont de Nemours and Company. His descendants, the du Pont family, have been one of the richest and most prominent American families since the 19th century, with generations of influential businessmen, politicians and philanthropists. In 1807, du Pont was elected a member of the American Philosophical Society in his adopted hometown of Philadelphia. Early life and education Du Pont was born 24 June 1771, in Paris, the son of Pierre Samuel du Pont de Nemours and Nicole-Charlotte Marie-Louise le Dée de Rencourt. His father was a political economist who had been elevated to the nobility in 1784 by letters patent granted by King Louis XVI, allowing him to carry the honorable ''de Nemours'' suffix. Growing up on his father's estate, "Bois des Fossés", near Égreville, young du Pont was enthusiastic about hi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calorimetry
In chemistry and thermodynamics, calorimetry () is the science or act of measuring changes in '' state variables'' of a body for the purpose of deriving the heat transfer associated with changes of its state due, for example, to chemical reactions, physical changes, or phase transitions under specified constraints. Calorimetry is performed with a calorimeter. Scottish physician and scientist Joseph Black, who was the first to recognize the distinction between heat and temperature, is said to be the founder of the science of calorimetry. Indirect calorimetry calculates heat that living organisms produce by measuring either their production of carbon dioxide and nitrogen waste (frequently ammonia in aquatic organisms, or urea in terrestrial ones), or from their consumption of oxygen. Lavoisier noted in 1780 that heat production can be predicted from oxygen consumption this way, using multiple regression. The dynamic energy budget theory explains why this procedure is corre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acid–base Reaction
In chemistry, an acid–base reaction is a chemical reaction that occurs between an acid and a base. It can be used to determine pH via titration. Several theoretical frameworks provide alternative conceptions of the reaction mechanisms and their application in solving related problems; these are called the acid–base theories, for example, Brønsted–Lowry acid–base theory. Their importance becomes apparent in analyzing acid–base reactions for gaseous or liquid species, or when acid or base character may be somewhat less apparent. The first of these concepts was provided by the French chemist Antoine Lavoisier, around 1776. – Table of discoveries attributes Antoine Lavoisier as the first to posit a scientific theory in relation to oxyacids. It is important to think of the acid–base reaction models as theories that complement each other. For example, the current Lewis model has the broadest definition of what an acid and base are, with the Brønsted–Lowry the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jacques-Louis David
Jacques-Louis David (; 30 August 1748 – 29 December 1825) was a French painter in the Neoclassicism, Neoclassical style, considered to be the preeminent painter of the era. In the 1780s, his cerebral brand of history painting marked a change in taste away from Rococo frivolity toward classical austerity, severity, and heightened feeling, which harmonized with the moral climate of the final years of the Ancien Régime. David later became an active supporter of the French Revolution and friend of Maximilien Robespierre (1758–1794), and was effectively a dictator of the arts under the French First Republic, French Republic. Imprisoned after Robespierre's fall from power, he aligned himself with yet another political regime upon his release: that of Napoleon, the First Consul of France. At this time he developed his Empire style, notable for its use of warm Venetian school (art), Venetian colours. After Napoleon's fall from Imperial power and the Bourbon revival, David exiled hims ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter. Under standard conditions, hydrogen is a gas of diatomic molecules with the chemical formula, formula , called dihydrogen, or sometimes hydrogen gas, molecular hydrogen, or simply hydrogen. Dihydrogen is colorless, odorless, non-toxic, and highly combustible. Stars, including the Sun, mainly consist of hydrogen in a plasma state, while on Earth, hydrogen is found as the gas (dihydrogen) and in molecular forms, such as in water and organic compounds. The most common isotope of hydrogen (H) consists of one proton, one electron, and no neutrons. Hydrogen gas was first produced artificially in the 17th century by the reaction of acids with metals. Henry Cavendish, in 1766–1781, identified hydrogen gas as a distinct substance and discovere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermochemistry
Thermochemistry is the study of the heat energy which is associated with chemical reactions and/or phase changes such as melting and boiling. A reaction may release or absorb energy, and a phase change may do the same. Thermochemistry focuses on the energy exchange between a system and its surroundings in the form of heat. Thermochemistry is useful in predicting reactant and product quantities throughout the course of a given reaction. In combination with entropy determinations, it is also used to predict whether a reaction is spontaneous or non-spontaneous, favorable or unfavorable. Endothermic reactions absorb heat, while exothermic reactions release heat. Thermochemistry coalesces the concepts of thermodynamics with the concept of energy in the form of chemical bonds. The subject commonly includes calculations of such quantities as heat capacity, heat of combustion, heat of formation, enthalpy, entropy, and free energy. Thermochemistry is one part of the broader field o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |