|
Laurolactam
Laurolactam is an organic compound from the group of macrocyclic lactams. Laurolactam is mainly used as a monomer in engineering plastics, such as nylon-12 and copolyamides.T. Schiffer, G. Oenbrink: ''Cyclododecanol, Cyclododecanone, and Laurolactam.'' In: ''Ullmann's Encyclopedia of Industrial Chemistry.'' Wiley-VCH, Weinheim 2002, . Synthesis Although a derivative of 12-amino dodecanoic acid, it is made from cyclododecatriene. The triene is hydrogenated to the saturated alkane, cyclododecane. For the production of laurolactam, cyclododecane is oxidized with air or oxygen in the presence of boric acid and transition metal salts (e.g. cobalt(II) acetate), obtaining a mixtureH.-J. Arpe: ''Industrielle Organische Chemie.'' 6., vollst. überarb. Aufl., Wiley-VCH Verlag, Weinheim, 2007, . of cyclododecanol and cyclododecanone. This mixture is quantitatively dehydrogenated on a copper contact catalyst to cyclododecanone and this reacted with hydroxylamine to cyclododecanone oxime. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyamide 12
Nylon 12 is a nylon polymer with the formula CH2)11C(O)NHsub>n. It is made from ω-aminolauric acid or laurolactam monomers that each have 12 carbons, hence the name ‘Nylon 12’. It is one of several nylon polymers. Synthesis Nylon 12 can be produced through two routes. The first being polycondensation of ω-aminolauric acid, a bifunctional monomer with one amine and one carboxylic acid group. n H2N(CH2)11CO2H → CH2)11CONHsub>n + n H2O The second route is ring-opening polymerization of laurolactam at 260-300˚C. Ring opening can be carried out by cationic or anionic initiators, although cationic initiators have not been used commercially due to the product being less stable and oxidized relatively quickly in comparison to those produced by activated anionic polymerization (monomer casting). Ring-opening polymerization is the preferred route for commercial production. :n CH2)11CONH → CH2)11CONHsub>n Properties Nylon 12 exhibits properties between short cha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclododecatriene
Cyclododecatrienes are cyclic trienes with the formula C12H18. Four isomers are known for 1,5,9-cyclododecatriene. The ''trans'',''trans'',''cis''-isomer is a precursor in the production of nylon-12. : Production The ''trans'',''trans'',''cis''-isomer is obtained by cyclotrimerization of butadiene catalyzed by a mixture of titanium tetrachloride and an organoaluminium co-catalyst. Production capacity in 1995 was 8000 tons. : As aforementioned, titanium catalysts predominantly produce the important ''cis'',''trans'',''trans''- isomer. The all-''trans'' isomer is, however, the product from nickel- and chromium-catalyzed trimerization reactions. The yield of cyclododecatriene through these methods is often greater than 80%. The principal side products are the dimers and oligomers of butadiene. Properties All of the isomers of 1,5,9-cyclododecatriene are colorless, possess typical terpene-like odors, and have low melting points. The all-''trans'' isomer melts at 34 °C while t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclododecane
Cyclododecane is an organic compound with the chemical formula (CH2)12. It is a waxy white solid at room temperature, and is soluble in nonpolar organic solvents. It is an intermediate of Nylon 12, polyesters, and synthetic lubricating oils. It is also used as a temporary binder to stabilise fragile objects or to seal water-sensitive parts; it slowly sublimates over days or weeks without leaving any residue. Uses It is a precursor to laurolactam, a precursor to the polymer Nylon 12. Cyclododecane is also an intermediate in production of flame retardants, detergents, and other chemicals. Cyclododecane is also used as a volatile binding medium, a temporary binder for sealing and conservation of friable and structurally weak materials, e.g. during excavation and transport of archaeological objects and in art restoration, e.g. to protect water-sensitive parts during cleaning. Due to its relatively slow evaporation in comparison with other volatile binding mediums the layer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclododecanone
Cyclododecanone is an organic compound with the formula (CH2)11CO. It is a cyclic ketone that exists as a white solid at room temperature. It is produced by the oxidation of cyclododecane via cyclododecanol. Cyclododecanone is mainly consumed as a precursor to 1,12-dodecanedioic acid and laurolactam, which are precursors to certain specialized nylons. Small amounts are also converted to cyclohexadecanone Cyclohexadecanone is an organic compound with the formula (CH2)15CO. It is a cyclic ketone, which is a minor component of the musk scent of the civet. Several related derivatives are also important in the fragrance industry, especially those ..., which is used in some fragrances.Johannes Panten and Horst Surburg "Flavors and Fragrances, 2. Aliphatic Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, 2015, Wiley-VCH, Weinheim. References Perfume ingredients Macrocycles Mammalian pheromones Cycloalkanones {{Ketone-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon- hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, hydrogen cyanide), are not classified as organic compounds and are considered inorganic. Other than those just named, little consensus exists among chemists on precisely which carbon-containing compounds are excluded, making any rigorous definition of an organic compound elusive. Although organic compounds make up only a small percentage of Earth's crust, they are of central importance because all known life is based on organic compounds. Livin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anhydrous Hydrogen Chloride
The compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colourless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hydrogen chloride gas and hydrochloric acid are important in technology and industry. Hydrochloric acid, the aqueous solution of hydrogen chloride, is also commonly given the formula HCl. Reactions Hydrogen chloride is a diatomic molecule, consisting of a hydrogen atom H and a chlorine atom Cl connected by a polar covalent bond. The chlorine atom is much more electronegative than the hydrogen atom, which makes this bond polar. Consequently, the molecule has a large dipole moment with a negative partial charge (δ−) at the chlorine atom and a positive partial charge (δ+) at the hydrogen atom. In part because of its high polarity, HCl is very soluble in water (and in other polar solvents). Upon contact, and HCl combine to form hydronium cations and ch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfuric Acid
Sulfuric acid ( American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formula . It is a colorless, odorless and viscous liquid that is miscible with water. Pure sulfuric acid does not exist naturally on Earth due to its strong affinity to water vapor; it is hygroscopic and readily absorbs water vapor from the air. Concentrated sulfuric acid is highly corrosive towards other materials, from rocks to metals, since it is an oxidant with powerful dehydrating properties. Phosphorus pentoxide is a notable exception in that it is not dehydrated by sulfuric acid, but to the contrary dehydrates sulfuric acid to sulfur trioxide. Upon addition of sulfuric acid to water, a considerable amount of heat is released; thus the reverse procedure of adding water to the acid should not be performed since the heat released ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rearrangement Reaction
In organic chemistry, a rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule, hence these reactions are usually intramolecular. In the example below, the substituent R moves from carbon atom 1 to carbon atom 2: :\underset\ce\ce\underset\ce\ce Intermolecular rearrangements also take place. A rearrangement is not well represented by simple and discrete electron transfers (represented by curved arrows in organic chemistry texts). The actual mechanism of alkyl groups moving, as in Wagner-Meerwein rearrangement, probably involves transfer of the moving alkyl group fluidly along a bond, not ionic bond-breaking and forming. In pericyclic reactions, explanation by orbital interactions give a better picture than simple discrete electron transfers. It is, nevertheless, possible to draw the curv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,4-Dioxane
1,4-Dioxane () is a heterocyclic organic compound, classified as an ether. It is a colorless liquid with a faint sweet odor similar to that of diethyl ether. The compound is often called simply dioxane because the other dioxane isomers ( 1,2- and 1,3-) are rarely encountered. Dioxane is used as a solvent for a variety of practical applications as well as in the laboratory, and also as a stabilizer for the transport of chlorinated hydrocarbons in aluminum containers.Wisconsin Department of Health Services (20131,4-Dioxane Fact Sheet Publication 00514. Accessed 2016-11-12. Synthesis Dioxane is produced by the acid-catalysed dehydration of diethylene glycol, which in turn is obtained from the hydrolysis of ethylene oxide. In 1985, the global production capacity for dioxane was between 11,000 and 14,000 tons. In 1990, the total U.S. production volume of dioxane was between 5,250 and 9,150 tons. Structure The dioxane molecule is centrosymmetric, meaning that it adopts a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
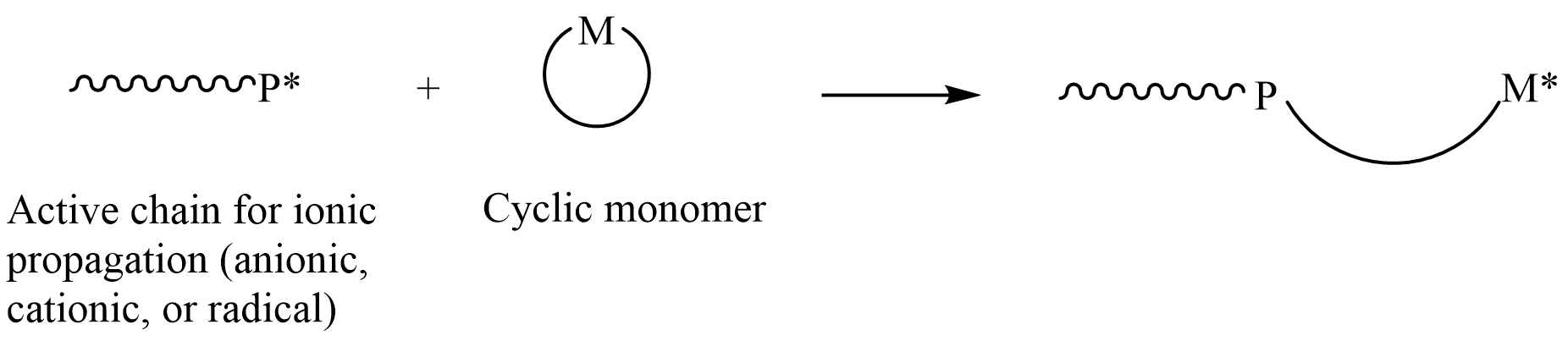
Ring-opening Polymerization
In polymer chemistry, ring-opening polymerization (ROP) is a form of chain-growth polymerization, in which the terminus of a polymer chain attacks cyclic monomers to form a longer polymer (see figure). The reactive center can be radical, anionic or cationic. Some cyclic monomers such as norbornene or cyclooctadiene can be polymerized to high molecular weight polymers by using metal catalysts. ROP is a versatile method for the synthesis of biopolymers. Ring-opening of cyclic monomers is often driven by the relief of bond-angle strain. Thus, as is the case for other types of polymerization, the enthalpy change in ring-opening is negative. Monomers Cyclic monomers that are amenable to ROP include epoxides, cyclic trisiloxanes, some lactones, lactides, cyclic carbonates, and amino acid N-carboxyanhydrides. Many strained cycloalkenes, e.g norbornene, are suitable monomers via ring-opening metathesis polymerization. History Ring-opening polymerization has been used since the b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |