|
L-rhamnose
Rhamnose (Rha, Rham) is a naturally occurring deoxy sugar. It can be classified as either a methyl-pentose or a 6-deoxy-hexose. Rhamnose predominantly occurs in nature in its L-form as L-rhamnose (6-deoxy-L-mannose). This is unusual, since most of the naturally occurring sugars are in D-form. Exceptions are the methyl pentoses L-fucose and L-rhamnose and the pentose L- arabinose. However, examples of naturally-occurring D-rhamnose are found in some species of bacteria, such as ''Pseudomonas aeruginosa'' and ''Helicobacter pylori''. Rhamnose can be isolated from buckthorn (''Rhamnus''), poison sumac, and plants in the genus ''Uncaria''. Rhamnose is also produced by microalgae belonging to class Bacillariophyceae (diatoms). Rhamnose is commonly bound to other sugars in nature. It is a common glycone component of glycosides from many plants. Rhamnose is also a component of the outer cell membrane of acid-fast bacteria in the ''Mycobacterium'' genus, which includes the organism ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Galactose Binding Lectin Domain
In molecular biology, the galactose binding lectin domain is a protein domain. It is found in many proteins including the lectin purified from sea urchin (''Anthocidaris crassispina'') eggs, SUEL. This lectin exists as a disulfide-linked homodimer of two subunits; the dimeric form is essential for hemagglutination activity. The sea urchin egg lectin (SUEL) forms a new class of lectins. Although SUEL was first isolated as a D-galactoside binding lectin, it was later shown that it binds to L-rhamnose preferentially. L-rhamnose and D-galactose share the same hydroxyl group orientation at C2 and C4 of the pyranose ring structure. A cysteine-rich domain (the galactose binding lectin domain) homologous to the SUEL protein has been identified in the following proteins: *Plant beta-galactosidases (lactases). *Mammalian latrophilin, the calcium independent receptor of alpha- latrotoxin (CIRL). The galactose-binding lectin domain is not required for alpha-latratoxin binding. **Human lat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deoxy Sugar
Deoxy sugars are sugars that have had a hydroxyl group replaced with a hydrogen atom. Examples include: * Deoxyribose, or 2-deoxy-D-ribose, a constituent of DNA * Fucose, or 6-deoxy-L-galactose, main component of fucoidan of brown algae, and present in N-linked glycans * Fuculose, or 6-deoxy-L- tagatose, one of the important components of avian influenza virus particles * Rhamnose, or 6-deoxy-L-mannose, present in plant glycosides In ''Escherichia coli'' bacteria, deoxyribose sugars are synthesized via two different pathways - one pathway involves aldol condensation, whereas the other pathway is conversion of a ribose sugar into a deoxyribose sugar by means of changes on the nucleotide or nucleoside level. Deoxyribose is synthesized through the reduction of ribose. Deoxyribose is derived from the same precursor as ribose being that the reduction of the sugar with the extra hydroxyl group results in the deoxy-sugar, which has its hydroxyl group replaced with a hydrogen atom. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mycobacterium
''Mycobacterium'' is a genus of over 190 species in the phylum Actinomycetota, assigned its own family, Mycobacteriaceae. This genus includes pathogens known to cause serious diseases in mammals, including tuberculosis (''Mycobacterium tuberculosis, M. tuberculosis'') and leprosy (''Mycobacterium leprae, M. leprae'') in humans. The Greek language, Greek prefix ''myco-'' means 'fungus', alluding to this genus' Mold (fungus), mold-like colony surfaces. Since this genus has cell walls with a waxy lipid-rich outer layer containing high concentrations of mycolic acid, acid-fast staining is used to emphasize their resistance to acids, compared to other cell types. Mycobacterial species are generally aerobic, non-motile, and capable of growing with minimal nutrition. The genus is divided based on each species' pigment production and growth rate. While most ''Mycobacterium'' species are non-pathogenic, the genus' characteristic complex cell wall contributes to evasion from host defenses. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rutinose
Rutinose is the disaccharide also known as 6-''O''-α-L- rhamnosyl-D-glucose (C12H22O10) that is present in some flavonoid glycosides. It is prepared from rutin by hydrolysis Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ... with the enzyme rhamnodiastase. References * Disaccharides Deoxy sugars {{Carbohydrate-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diol
A diol is a chemical compound containing two hydroxyl groups ( groups). An aliphatic diol may also be called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified. They are used as protecting groups of carbonyl groups, making them essential in synthesis of organic chemistry. The most common industrial diol is ethylene glycol. Examples of diols in which the hydroxyl functional groups are more widely separated include 1,4-butanediol and propylene-1,3-diol, or beta propylene glycol, . Synthesis of classes of diols Geminal diols A geminal diol has two hydroxyl groups bonded to the same atom. These species arise by hydration of the carbonyl compounds. The hydration is usually unfavorable, but a notable exception is formaldehyde which, in water, exists in equilibrium with methanediol H2C(OH)2. Another example is (F3C)2C(OH)2, the hydrated form of hexafluoroacetone. Many gem-diols undergo further condensation to give dimer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycerol
Glycerol () is a simple triol compound. It is a colorless, odorless, sweet-tasting, viscous liquid. The glycerol backbone is found in lipids known as glycerides. It is also widely used as a sweetener in the food industry and as a humectant in pharmaceutical formulations. Because of its three hydroxyl groups, glycerol is miscible with water and is Hygroscopy, hygroscopic in nature. Modern use of the word glycerine (alternatively spelled glycerin) refers to commercial preparations of less than 100% purity, typically 95% glycerol. Structure Although chirality, achiral, glycerol is prochirality, prochiral with respect to reactions of one of the two primary alcohols. Thus, in substituted derivatives, the Glycerophospholipid#Nomenclature and stereochemistry, stereospecific numbering labels the molecule with a ''sn''- prefix before the stem name of the molecule. Production Natural sources Glycerol is generally obtained from plant and animal sources where it occurs in triglycerides, est ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
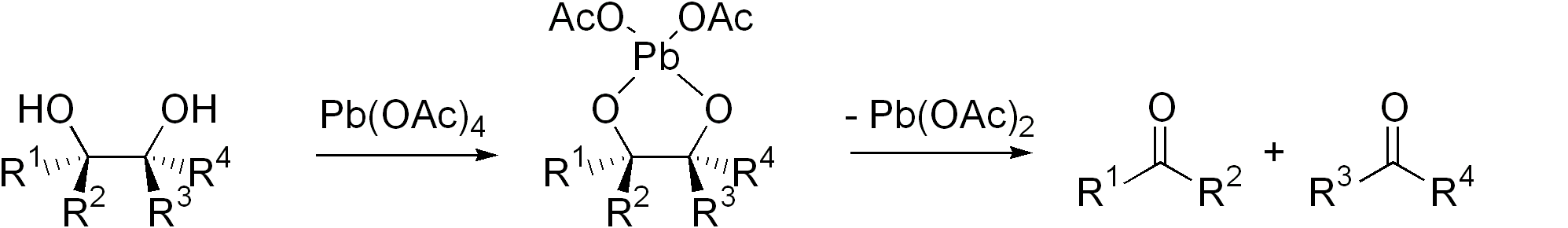
Diol Cleavage
Glycol cleavage is a specific type of organic chemistry oxidation. The carbon–carbon bond in a vicinal diol (glycol) is cleaved and instead the two oxygen atoms become double-bonded to their respective carbon atoms. Depending on the substitution pattern in the diol, these carbonyls will be ketones and/or aldehydes. Glycol cleavage is an important for determining the structures of sugars. After cleavage of the glycol, the ketone and aldehyde fragments can be inspected and the location of the former hydroxyl groups ascertained. Reagents Iodine-based reagents such as periodic acid (HIO4) and (diacetoxyiodo)benzene (PhI(OAc)2) are commonly used. Another reagent is lead tetraacetate (Pb(OAc)4). These I- and Pb-based methods are called the Malaprade reaction and Criegee oxidation, respectively. The former is favored for aqueous solutions, the latter for nonaqueous solutions. Cyclic intermediate are invariably invoked. The ring then fragments, with cleavage of the carbon–carb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vicinal (chemistry)
In chemistry the descriptor vicinal (from Latin ''vicinus'' = neighbor), abbreviated ''vic'', is a descriptor that identifies two functional groups as bonded to two adjacent carbon atoms (i.e., in a 1,2-relationship). It may arise from vicinal difunctionalization. Relation of atoms in a molecule For example, the molecule 2,3-dibromobutane carries two vicinal bromine Bromine is a chemical element; it has chemical symbol, symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between th ... atoms and 1,3-dibromobutane does not. Mostly, the use of the term vicinal is restricted to two identical functional groups. Likewise in a ''gem-''dibromide the prefix ''gem'', an abbreviation of '' geminal'', signals that both bromine atoms are bonded to the same carbon atom (i.e., in a 1,1-relationship). For example, 1,1-dibromobutane is geminal. While comparativel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Periodate
Periodate ( ) is an anion composed of iodine and oxygen. It is one of a number of oxyanions of iodine and is the highest in the series, with iodine existing in oxidation state +7. Unlike other perhalogenates, such as perchlorate, it can exist in two forms: metaperiodate and orthoperiodate . In this regard it is comparable to the tellurate ion from the adjacent group. It can combine with a number of counter ions to form periodates, which may also be regarded as the salts of periodic acid. Periodates were discovered by Heinrich Gustav Magnus and C. F. Ammermüller; who first synthesised periodic acid in 1833. Synthesis Classically, periodate was most commonly produced in the form of sodium hydrogen periodate (). This is commercially available, but can also be produced by the oxidation of iodates with chlorine and sodium hydroxide. Or, similarly, from iodides by oxidation with bromine and sodium hydroxide: :\overset + Cl2 + 4 NaOH -> Na3H2IO6 + 2NaCl + H2O :NaI + 4 Br2 + 10 NaOH ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Formaldehyde
Formaldehyde ( , ) (systematic name methanal) is an organic compound with the chemical formula and structure , more precisely . The compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde. It is stored as aqueous solutions (formalin), which consists mainly of the hydrate CH2(OH)2. It is the simplest of the aldehydes (). As a precursor to many other materials and chemical compounds, in 2006 the global production of formaldehyde was estimated at 12 million tons per year. It is mainly used in the production of industrial resins, e.g., for particle board and coatings. Formaldehyde also occurs naturally. It is derived from the degradation of serine, dimethylglycine, and lipids. Demethylases act by converting N-methyl groups to formaldehyde. Formaldehyde is classified as a group 1 carcinogen and can cause respiratory and skin irritation upon exposure. Forms Formaldehyde is more complicated than many simple carbon compounds in that i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycan
The terms glycans and polysaccharides are defined by IUPAC as synonyms meaning "compounds consisting of a large number of monosaccharides linked glycosidically". However, in practice the term glycan may also be used to refer to the carbohydrate portion of a glycoconjugate, such as a glycoprotein, glycolipid, or a proteoglycan, even if the carbohydrate is only an oligosaccharide. Glycans usually consist solely of O-glycosidic linkages of monosaccharides. For example, cellulose is a glycan (or, to be more specific, a glucan) composed of β-1,4-linked D-glucose, and chitin is a glycan composed of β-1,4-linked ''N''-acetyl-D-glucosamine. Glycans can be homo- or heteropolymers of monosaccharide residues, and can be linear or branched. Interactions with proteins Glycans can be found attached to proteins as in glycoproteins and proteoglycans. In general, they are found on the exterior surface of cells. ''O''- and ''N''-linked glycans are very common in eukaryotes but may ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |