|
L-760790
L-760,790 is an experimental drug from the substituted tryptamine family, which acts as a selective agonist for the 5-HT1D serotonin receptor 5-HT receptors, 5-hydroxytryptamine receptors, or serotonin receptors, are a group of G protein-coupled receptor and ligand-gated ion channels found in multiple tissues including the central and peripheral nervous systems. They mediate both ex ..., with around 62x selectivity for 5-HT1D over the closely related 5-HT1B receptor. References 5-HT1B agonists 5-HT1D agonists Benzylamines Indoles Pyrrolidines Triazoles Secondary amines {{nervous-system-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-HT1D Receptor
5-hydroxytryptamine (serotonin) receptor 1D, also known as HTR1D, is a 5-HT receptor, but also denotes the human gene encoding it. 5-HT1D acts on the central nervous system, and affects locomotion and anxiety. It also induces vasoconstriction in the brain. Tissue distribution 5HT1D receptors are found at low levels in the basal ganglia (globus pallidus, substantia nigra, caudate putamen), the hippocampus, and in the cortex. Structure 5HT1D receptor is a G protein linked receptor that activates an intracellular messenger cascade to produce an inhibitory response by decreasing cellular levels of cAMP. The 5HT1D is a 7-TM receptor. A large intercellular loop between TM-5 and TM-6 is believed to be associated with coupling to a second messenger. Agonists might bind in a manner that utilizes an aspartate residue in TM-3 and residues in the TM-4, TM-5 and TM-6. A human clone containing an intronless open reading frame was found to encode 377 amino acids of the 5HT1D receptor. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-HT1D Agonists
5-hydroxytryptamine (serotonin) receptor 1D, also known as HTR1D, is a 5-HT receptor, but also denotes the human gene encoding it. 5-HT1D acts on the central nervous system, and affects animal locomotion, locomotion and anxiety. It also induces vasoconstriction in the brain. Tissue distribution 5HT1D receptors are found at low levels in the basal ganglia (globus pallidus, substantia nigra, caudate putamen), the hippocampus, and in the cortex. Structure 5HT1D receptor is a G protein linked receptor that activates an intracellular messenger cascade to produce an inhibitory response by decreasing cellular levels of cAMP. The 5HT1D is a G protein–coupled receptor, 7-TM receptor. A large intercellular loop between TM-5 and TM-6 is believed to be associated with coupling to a second messenger. Agonists might bind in a manner that utilizes an aspartate residue in TM-3 and residues in the TM-4, TM-5 and TM-6. A human clone containing an intronless open reading frame was found to en ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substituted Tryptamine
Substituted tryptamines, or simply tryptamines, also known as serotonin analogues (i.e., 5-hydroxytryptamine analogues), are organic compounds which may be thought of as being derived from tryptamine itself. The molecular structures of all tryptamines contain an indole ring, joined to an amino group, amino (NH2) group via an ethyl (−CH2–CH2−) side chain, sidechain. In substituted tryptamines, the indole ring, sidechain, and/or amino group are modified by substituting another group for one of the hydrogen (H) atoms. Well-known tryptamines include serotonin, an important neurotransmitter, and melatonin, a hormone involved in regulating the sleep-wake cycle. Tryptamine alkaloids are found in fungi, plants and animals; and sometimes used by humans for the neurological or psychotropic effects of the substance. Prominent examples of tryptamine alkaloids include psilocybin (from "psilocybin mushrooms") and dimethyltryptamine, DMT. In South America, dimethyltryptamine is obtained f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serotonin Receptor
5-HT receptors, 5-hydroxytryptamine receptors, or serotonin receptors, are a group of G protein-coupled receptor and ligand-gated ion channels found in multiple tissues including the central and peripheral nervous systems. They mediate both excitatory and inhibitory neurotransmission. The serotonin (i.e., 5-hydroxytryptamine, hence "5-HT") receptors are activated by the neurotransmitter serotonin, which acts as their natural ligand. The serotonin receptors modulate the release of many neurotransmitters, including glutamate, GABA, dopamine, epinephrine / norepinephrine, and acetylcholine, as well as many hormones, including oxytocin, prolactin, vasopressin, cortisol, corticotropin, and substance P, among others. Serotonin receptors influence various biological and neurological processes such as aggression, anxiety, appetite, cognition, learning, memory, mood, nausea, sleep, and thermoregulation. They are the target of a variety of pharmaceutical and recreational drugs, inclu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-HT1B Receptor
5-hydroxytryptamine receptor 1B also known as the 5-HT1B receptor is a protein that in humans is encoded by the ''HTR1B'' gene. The 5-HT1B receptor is a 5-HT receptor subtype. Tissue distribution and function 5-HT1B receptors are widely distributed throughout the central nervous system with the highest concentrations found in the frontal cortex, basal ganglia, striatum, and the hippocampus. The function of the 5-HT1B receptor differs depending upon its location. In the frontal cortex, it is believed to act as a terminal receptor inhibiting the release of dopamine. In the basal ganglia and the striatum, evidence suggests 5-HT signaling acts on an autoreceptor, inhibiting the release of serotonin and decreasing glutamatergic transmission by reducing miniature excitatory postsynaptic potential (mEPSP) frequency, respectively. In the hippocampus, a recent study has demonstrated that activation of postsynaptic 5-HT1B heteroreceptors produces a facilitation in excitatory synapt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzylamines
Benzylamine, also known as phenylmethylamine, is an organic chemical compound with the condensed structural formula C6H5CH2NH2 (sometimes abbreviated as PhCH2NH2 or BnNH2). It consists of a benzyl group, C6H5CH2, attached to an amine functional group, NH2. This colorless water-soluble liquid is a common precursor in organic chemistry and used in the industrial production of many pharmaceuticals. The hydrochloride salt was used to treat motion sickness on the Mercury-Atlas 6 mission in which NASA astronaut John Glenn became the first American to orbit the Earth. Manufacturing Benzylamine can be produced by several methods, the main industrial route being the reaction of benzyl chloride and ammonia. It is also produced by the reduction of benzonitrile and reductive amination of benzaldehyde, both done over Raney nickel. : It was first produced accidentally by Rudolf Leuckart in the reaction of benzaldehyde with formamide in a process now known as the Leuckart reaction. Bio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indoles
Indole is an organic compound with the formula . Indole is classified as an aromatic Heterocyclic compound, heterocycle. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indoles are derivatives of indole where one or more of the hydrogen atoms have been replaced by substituent groups. Indoles are widely distributed in nature, most notably as amino acid tryptophan and neurotransmitter serotonin. General properties and occurrence Indole is a solid at room temperature. It occurs naturally in human feces and has an intense fecal odor. At very low concentrations, however, it has a flowery smell, and is a constituent of many perfumes. It also occurs in coal tar. It has been identified in cannabis. It is the main volatile compound in stinky tofu. When indole is a substituent on a larger molecule, it is called an ''indolyl group'' by systematic nomenclature. Indole undergoes electrophilic substitution, mainly at position ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrrolidines
Pyrrolidine, also known as tetrahydropyrrole, is an organic compound with the molecular formula (CH2)4NH. It is a cyclic secondary amine, also classified as a saturated heterocycle. It is a colourless liquid that is miscible with water and most organic solvents. It has a characteristic odor that has been described as "ammoniacal, fishy, shellfish-like". In addition to pyrrolidine itself, many substituted pyrrolidines are known. Production and synthesis Industrial production Pyrrolidine is prepared industrially by the reaction of 1,4-butanediol and ammonia at a temperature of 165–200 °C and a pressure of 17–21 MPa in the presence of a cobalt- and nickel oxide catalyst, which is supported on alumina. : The reaction is carried out in the liquid phase in a continuous tube- or tube bundle reactor, which is operated in the cycle gas method. The catalyst is arranged as a fixed-bed and the conversion is carried out in the downflow mode. The product is obtained after m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
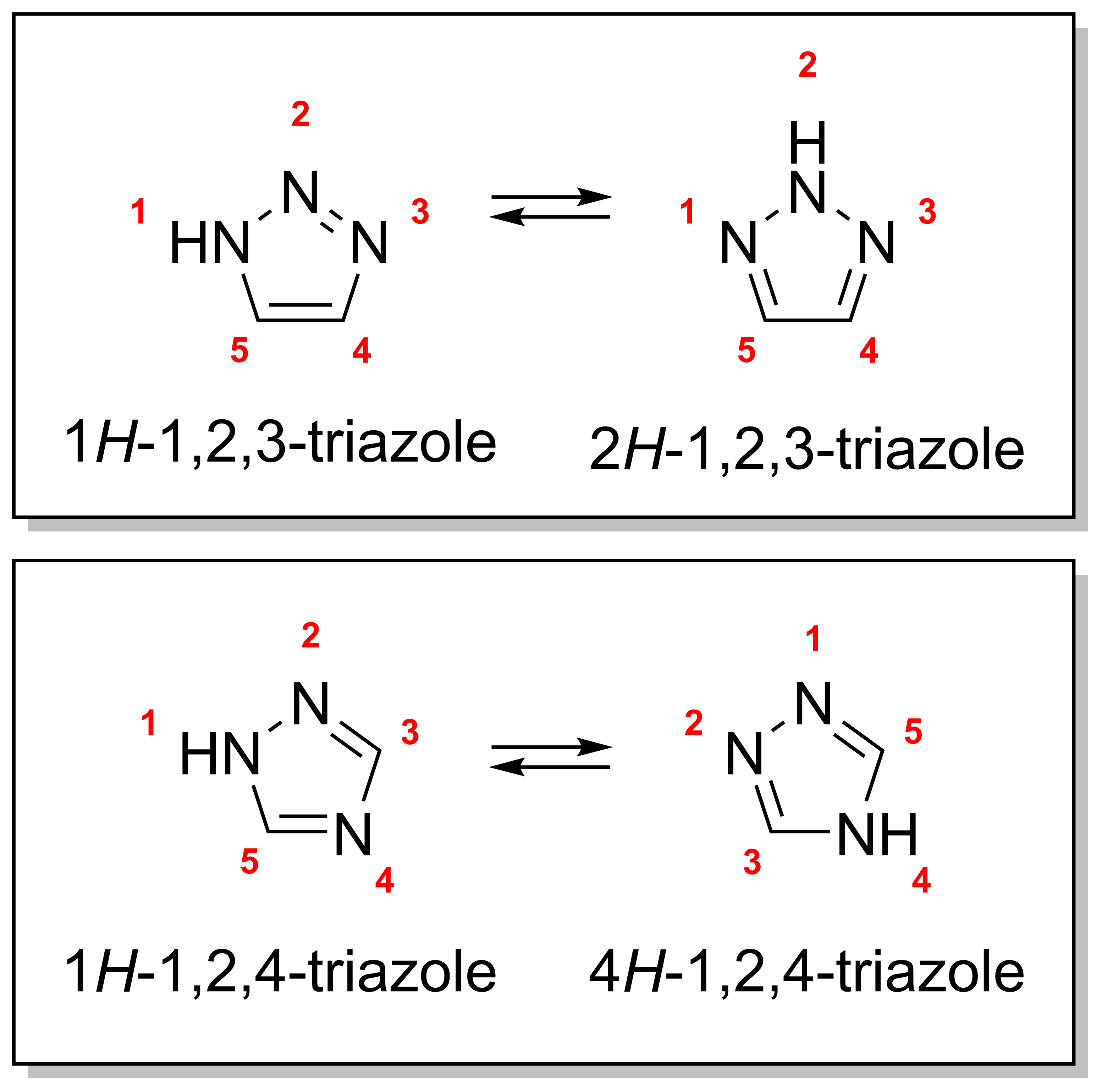
Triazoles
A triazole is a heterocyclic compound featuring a five-membered ring of two carbon atoms and three nitrogen atoms with molecular formula C2H3N3. Triazoles exhibit substantial Isomer, isomerism, depending on the positioning of the nitrogen atoms within the ring. Many triazoles are versatile, biologically active compounds commonly used as fungicides and plant retardants. However, triazoles are also useful in bioorthogonal chemistry, because the large number of nitrogen atoms causes triazoles to react similar to Azide, azides. Lastly, the many free lone pairs in triazoles make them useful as coordination compounds, although not typically as Piano stool complex, haptic ligands. Isomerism There are four triazole isomers, which are conventionally divided into two pairs of Tautomer, tautomers. In the 1,2,3-Triazole, 1,2,3-triazoles, the three nitrogen atoms are adjacent; in the 1,2,4-Triazole, 1,2,4-triazoles, an interstitial carbon separates out one nitrogen atom. Each category ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |