|
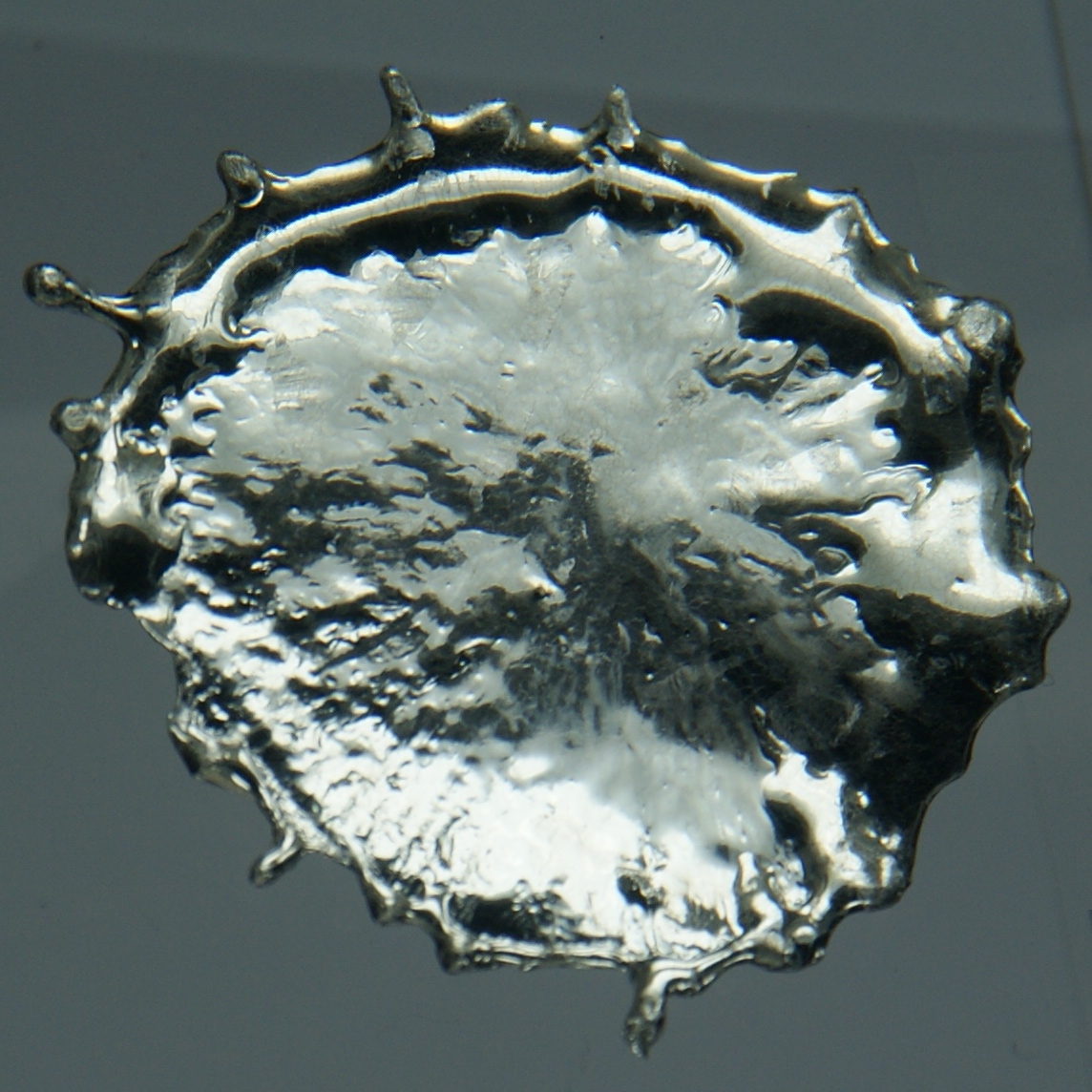
Kesterite
Kësterite is a sulfide mineral with a chemical formula of . In its lattice structure, zinc and iron atoms share the same lattice sites. Kesterite is the Zn-rich variety whereas the Zn-poor form is called ferrokesterite or stannite. Owing to their similarity, kesterite is sometimes called isostannite. The synthetic form of kesterite is abbreviated as CZTS (from copper zinc tin sulfide). The name kesterite is sometimes extended to include this synthetic material and also CZTSe, which contains selenium instead of sulfur. Occurrence Kesterite was first described in 1958 in regard to an occurrence in the Kester deposit (and the associated locality) in Ynnakh Mountain, Yana basin, Yakutia, Russia, where it was discovered. It is usually found in quartz-sulfide hydrothermal veins associated with tin ore deposits. Associated minerals include arsenopyrite, stannoidite, chalcopyrite, chalcocite, sphalerite and tennantite. Stannite and kesterite occur together in the Ivigtut cryolite de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CZTS
Copper zinc tin sulfide (CZTS) is a quaternary semiconducting compound which has received increasing interest since the late 2000s for applications in thin film solar cells. The class of related materials includes other I2-II-IV-VI4 such as copper zinc tin selenide (CZTSe) and the sulfur-selenium alloy CZTSSe. CZTS offers favorable optical and electronic properties similar to CIGS (copper indium gallium selenide), making it well suited for use as a thin-film solar cell absorber layer, but unlike CIGS (or other thin films such as CdTe), CZTS is composed of only abundant and non-toxic elements. Concerns with the price and availability of indium in CIGS and tellurium in CdTe, as well as toxicity of cadmium have been a large motivator to search for alternative thin film solar cell materials. The power conversion efficiency of CZTS is still considerably lower than CIGS and CdTe, with laboratory cell records of 11.0 % for CZTS and 12.6 % for CZTSSe . Crystal structure CZTS is a I2-II- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CZTSe
Copper zinc tin sulfide (CZTS) is a quaternary semiconducting compound which has received increasing interest since the late 2000s for applications in thin film solar cells. The class of related materials includes other I2-II-IV-VI4 such as copper zinc tin selenide (CZTSe) and the sulfur-selenium alloy CZTSSe. CZTS offers favorable optical and electronic properties similar to CIGS (copper indium gallium selenide), making it well suited for use as a thin-film solar cell absorber layer, but unlike CIGS (or other thin films such as CdTe), CZTS is composed of only abundant and non-toxic elements. Concerns with the price and availability of indium in CIGS and tellurium in CdTe, as well as toxicity of cadmium have been a large motivator to search for alternative thin film solar cell materials. The power conversion efficiency of CZTS is still considerably lower than CIGS and CdTe, with laboratory cell records of 11.0 % for CZTS and 12.6 % for CZTSSe . Crystal structure CZTS is a I2-II-I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ynnakh Mountain
Ynnakh Mountain, also known as Arga Ynnakh Khaya (), Gora Ulakhan Ynnakh () and as Mother Mountain (), is a mountain in Verkhoyansky District, Yakutia, Russian Federation. The mountain has been classified as a natural monument of Russia with number 1420068. It is an important mountain in Yakut culture, where the word "Ynnakh" comes from , meaning scary, creepy. Geography Ynnakh Mountain is a granite massif located north of the Yana Plateau between the Yana River and the Adycha, a right hand tributary of the Yana. The mountain rises at the western limit of the Chersky Range, near the eastern foothills of the Verkhoyansk Range, a few miles to the southeast of Ese-Khayya. The height of the summit is according to the Operational Navigation Chart. According to other sources it is high. The mineral kesterite is found in the mountain. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfide Mineral
The sulfide minerals are a class of minerals containing sulfide (S2−) or disulfide () as the major anion. Some sulfide minerals are economically important as metal ores. The sulfide class also includes the selenide mineral, selenides, the telluride mineral, tellurides, the arsenide mineral, arsenides, the antimonide mineral, antimonides, the bismuthinides, the sulfarsenides and the sulfosalts.http://www.minerals.net/mineral/sort-met.hod/group/sulfgrp.htm Minerals.net Dana Classification, SulfidesKlein, Cornelis and Cornelius S. Hurlbut, Jr., 1986, ''Manual of Mineralogy'', Wiley, 20th ed., pp 269-293 Sulfide minerals are inorganic compounds. Minerals Common or important examples include: * Acanthite *Chalcocite *Bornite *Galena *Sphalerite *Chalcopyrite *Pyrrhotite *Millerite *Pentlandite *Covellite *Cinnabar *Realgar *Orpiment *Stibnite *Pyrite *Marcasite *Molybdenite Sulfarsenides: *Cobaltite *Arsenopyrite *Gersdorffite Sulfosalts: *Pyrargyrite *Pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfide Minerals
The sulfide minerals are a class of minerals containing sulfide (S2−) or disulfide () as the major anion. Some sulfide minerals are economically important as metal ores. The sulfide class also includes the selenides, the tellurides, the arsenides, the antimonides, the bismuthinides, the sulfarsenides and the sulfosalts.http://www.minerals.net/mineral/sort-met.hod/group/sulfgrp.htm Minerals.net Dana Classification, SulfidesKlein, Cornelis and Cornelius S. Hurlbut, Jr., 1986, ''Manual of Mineralogy'', Wiley, 20th ed., pp 269-293 Sulfide minerals are inorganic compounds. Minerals Common or important examples include: * Acanthite * Chalcocite * Bornite *Galena *Sphalerite *Chalcopyrite *Pyrrhotite * Millerite *Pentlandite * Covellite *Cinnabar * Realgar *Orpiment * Stibnite *Pyrite * Marcasite * Molybdenite Sulfarsenides: * Cobaltite * Arsenopyrite * Gersdorffite Sulfosalts: * Pyrargyrite * Proustite * Tetrahedrite * Tennantite * Enargite * B ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfide Mineral
The sulfide minerals are a class of minerals containing sulfide (S2−) or disulfide () as the major anion. Some sulfide minerals are economically important as metal ores. The sulfide class also includes the selenide mineral, selenides, the telluride mineral, tellurides, the arsenide mineral, arsenides, the antimonide mineral, antimonides, the bismuthinides, the sulfarsenides and the sulfosalts.http://www.minerals.net/mineral/sort-met.hod/group/sulfgrp.htm Minerals.net Dana Classification, SulfidesKlein, Cornelis and Cornelius S. Hurlbut, Jr., 1986, ''Manual of Mineralogy'', Wiley, 20th ed., pp 269-293 Sulfide minerals are inorganic compounds. Minerals Common or important examples include: * Acanthite *Chalcocite *Bornite *Galena *Sphalerite *Chalcopyrite *Pyrrhotite *Millerite *Pentlandite *Covellite *Cinnabar *Realgar *Orpiment *Stibnite *Pyrite *Marcasite *Molybdenite Sulfarsenides: *Cobaltite *Arsenopyrite *Gersdorffite Sulfosalts: *Pyrargyrite *Pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tin Minerals
Tin is a chemical element; it has symbol Sn () and atomic number 50. A silvery-colored metal, tin is soft enough to be cut with little force, and a bar of tin can be bent by hand with little effort. When bent, a bar of tin makes a sound, the so-called "tin cry", as a result of twinning in tin crystals. Tin is a post-transition metal in group 14 of the periodic table of elements. It is obtained chiefly from the mineral cassiterite, which contains stannic oxide, . Tin shows a chemical similarity to both of its neighbors in group 14, germanium and lead, and has two main oxidation states, +2 and the slightly more stable +4. Tin is the 49th most abundant element on Earth, making up 0.00022% of its crust, and with 10 stable isotopes, it has the largest number of stable isotopes in the periodic table, due to its magic number of protons. It has two main allotropes: at room temperature, the stable allotrope is β-tin, a silvery-white, malleable metal; at low temperatures it is le ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron(II) Minerals
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's outer and inner core. It is the fourth most abundant element in the Earth's crust, being mainly deposited by meteorites in its metallic state. Extracting usable metal from iron ores requires kilns or furnaces capable of reaching , about 500 °C (900 °F) higher than that required to smelt copper. Humans started to master that process in Eurasia during the 2nd millennium BC and the use of iron tools and weapons began to displace copper alloys – in some regions, only around 1200 BC. That event is considered the transition from the Bronze Age to the Iron Age. In the modern world, iron alloys, such as steel, stainless steel, cast iron and special steels, are by far the most common industrial metals, due to their mechanical pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper Minerals
Copper is a chemical element; it has Chemical symbol, symbol Cu (from Latin ) and atomic number 29. It is a soft, malleable, and ductility, ductile metal with very high thermal conductivity, thermal and electrical conductivity. A freshly exposed surface of pure copper has a Copper (color), pinkish-orange color. Copper is used as a conductor of heat and electricity, as a building material#Metal, building material, and as a constituent of various metal alloys, such as sterling silver used in jewelry, cupronickel used to make marine hardware and coins, and constantan used in strain gauges and thermocouples for temperature measurement. Copper is one of the few metals that can occur in nature in a directly usable, unalloyed metallic form. This means that copper is a native metal. This led to very early human use in several regions, from . Thousands of years later, it was the first metal to be Smelting, smelted from sulfide ores, ; the first metal to be cast into a shape in a mold, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chalcocite
Chalcocite (), copper(I) sulfide (Cu2S), is an important copper ore mineral. It is opaque and dark gray to black, with a metallic luster. It has a hardness of 2.5–3 on the Mohs scale. It is a sulfide with a monoclinic crystal system. The term ''chalcocite'' from the Greek ''khalkos'', meaning "copper". It is also known as redruthite, vitreous copper, or copper-glance. Occurrence Chalcocite is sometimes found as a primary vein mineral in hydrothermal veins. However, most chalcocite occurs in the supergene enriched environment below the oxidation zone of copper deposits as a result of the leaching of copper from the oxidized minerals. It is also often found in sedimentary rocks. It has been mined for centuries and is one of the most profitable copper ores. The reasons for this is its high copper content (66.7% atomic ratio and nearly 80% by weight) and the ease at which copper can be separated from sulfur. Since chalcocite is a secondary mineral that forms from the al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solid Solution
A solid solution, a term popularly used for metals, is a homogeneous mixture of two compounds in solid state and having a single crystal structure. Many examples can be found in metallurgy, geology, and solid-state chemistry. The word "solution" is used to describe the intimate mixing of components at the atomic level and distinguishes these homogeneous materials from physical mixtures of components. Two terms are mainly associated with solid solutions – ''solvents'' and ''solutes,'' depending on the relative abundance of the atomic species. In general if two compounds are isostructural then a solid solution will exist between the end members (also known as parents). For example sodium chloride and potassium chloride have the same cubic crystal structure so it is possible to make a pure compound with any ratio of sodium to potassium (Na1-xKx)Cl by dissolving that ratio of NaCl and KCl in water and then evaporating the solution. A member of this family is sold under the bra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |