|
Joint Committee For Traceability In Laboratory Medicine
The Joint Committee for Traceability in Laboratory Medicine or JCTLM is collaboration between the International Bureau of Weights and Measures (BIPM), the International Federation for Clinical Chemistry and Laboratory Medicine (IFCC), and the International Laboratory Accreditation Cooperation (ILAC). The goal of the JCTLM is to provide a worldwide platform to promote and give guidance on internationally recognized and accepted equivalence of measurements in laboratory medicine and traceability to appropriate measurement standards. See also * Good laboratory practice (GLP) * Institute for Reference Materials and Measurements (IRMM) * Reference range * Reference values A reference is a relationship between Object (philosophy), objects in which one object designates, or acts as a means by which to connect to or link to, another object. The first object in this relation is said to ''refer to'' the second object. ... References External links Joint Committee for Traceabili ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
International Bureau Of Weights And Measures
The International Bureau of Weights and Measures (, BIPM) is an List of intergovernmental organizations, intergovernmental organisation, through which its 64 member-states act on measurement standards in areas including chemistry, ionising radiation, physical metrology, as well as the International System of Units (SI) and Coordinated Universal Time (UTC). It is headquartered in the Pavillon de Breteuil in Saint-Cloud, near Paris, France. The organisation has been referred to as IBWM (from its name in English) in older literature. Function The BIPM has the mandate to provide the basis for a single, coherent system of measurements throughout the world, traceable to the International System of Units, International System of Units (SI). This task takes many forms, from direct dissemination of units to coordination through international comparisons of national measurement standards (as in electricity and ionising radiation). Following consultation, a draft version of the BIPM Work ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IFCC
The International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) is a global professional association that promotes the fields of clinical chemistry and laboratory medicine. It was established in Paris in 1952 as the International Association of Clinical Biochemists to organize the various national societies of these fields and is based in Milan, Italy. The IFCC's aims are to set global standards, support and educate its members, and provide conferences and other gatherings for sharing knowledge among the global laboratory medicine community. IFCC members fall into three groups: national societies of clinical chemistry and laboratory medicine, corporations, and affiliate international or national societies involved in laboratory medicine. As of 2023, these members represented more than 45,000 individual clinical chemists, laboratory scientists, and laboratory physicians. Structure and organization The IFCC is governed by a council consisting of representative ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
International Laboratory Accreditation Cooperation
The International Laboratory Accreditation Cooperation or ILAC started as a conference in 1977 to develop international cooperation for facilitating trade by promoting the acceptance of accredited test and calibration results. In 1996, ILAC became a formal cooperation with a charter to establish a network of mutual recognition agreements among accreditation bodies that would fulfil this aim. The ultimate aim of the ILAC is increased use and acceptance by industry as well as government of the results from accredited laboratories, including results from laboratories in other countries. In this way, the free-trade goal of a 'product tested once and accepted everywhere' can be realised. See also * Accreditation * Good laboratory practice (GLP) * Institute for Reference Materials and Measurements (IRMM) * International Federation of Clinical Chemistry and Laboratory Medicine * ISO/IEC 17025 * Joint Committee for Traceability in Laboratory Medicine * Reference range * Reference ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Good Laboratory Practice
The Principles of Good Laboratory Practice (GLP) establish rules and criteria for a quality system that oversees the organizational processes and conditions in which non-clinical (non-pharmaceutical) health and environmental safety–or simply toxicology–studies are planned, conducted, monitored, recorded, reported, and archived. These principles apply to the toxicity testing of chemicals in commerce, to ensure the quality and integrity of the safety data submitted by manufacturers to regulatory authorities globally. History The historical events leading to the proposal of the Good Laboratory Practice (GLP) regulations are crucial for understanding why these regulations are important to improve the quality and integrity of chemical safety data. They were developed in response to concerns about the reliability of toxicity data from industry. The GLP regulations aim to standardize procedures and practices to ensure accurate, reliable, and traceable safety data. GLP was first introd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Institute For Reference Materials And Measurements
The Institute for Reference Materials and Measurements (IRMM), located in Geel, Belgium, is one of the seven institutes of the Joint Research Centre (JRC), a Directorate-General of the European Commission (EC). The IRMM promotes a common and reliable European measurement system in support of European Union policies. The institute works on the production and dissemination of quality assurance tools, such as validated methods, reference materials, reference measurements, interlaboratory comparisons and training in best practices and experience in all areas where IRMM is working. The institute was founded in 1957 under the Treaties of Rome and started operation in 1960 under the name of the Central Bureau for Nuclear Measurements (CBNM). In 1986 the programme for a Community Bureau of Reference was announced. In 1993 the institute was renamed to reflect the new mission of the institute, which covers a wide range of measurement problems from food safety to environmental pollution. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reference Range
In medicine and health-related fields, a reference range or reference interval is the range or the interval of values that is deemed normal for a physiological measurement in healthy persons (for example, the amount of creatinine in the blood, or the partial pressure of oxygen). It is a basis for comparison for a physician or other health professional to interpret a set of test results for a particular patient. Some important reference ranges in medicine are reference ranges for blood tests and reference ranges for urine tests. The standard definition of a reference range (usually referred to if not otherwise specified) originates in what is most prevalent in a reference group taken from the general (i.e. total) population. This is the general reference range. However, there are also ''optimal health ranges'' (ranges that appear to have the optimal health impact) and ranges for particular conditions or statuses (such as pregnancy reference ranges for hormone levels). Value ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
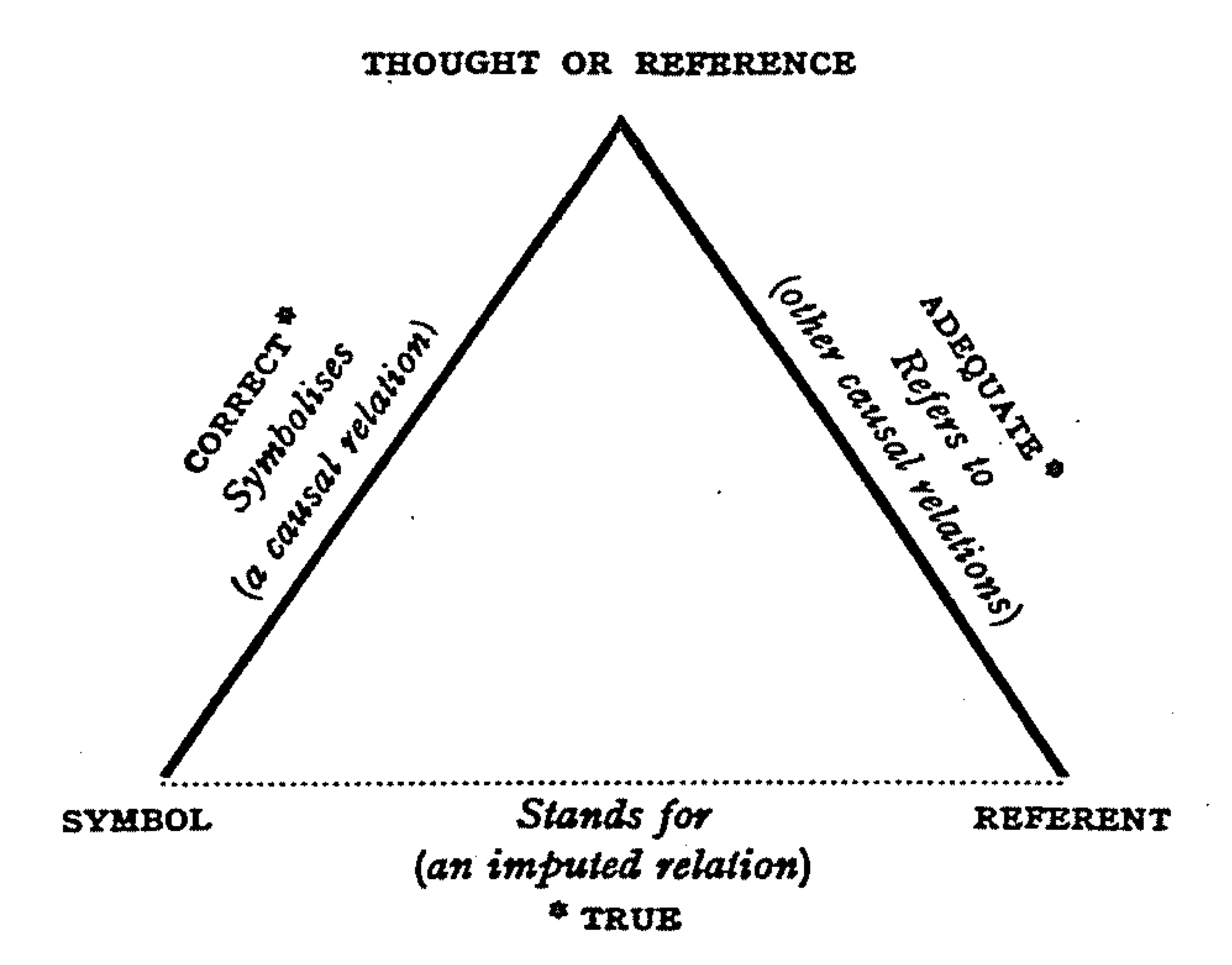
Reference Values
A reference is a relationship between Object (philosophy), objects in which one object designates, or acts as a means by which to connect to or link to, another object. The first object in this relation is said to ''refer to'' the second object. It is called a ''name'' for the second object. The next object, the one to which the first object refers, is called the ''referent'' of the first object. A name is usually a phrase or expression, or some other Symbol, symbolic representation. Its referent may be anything – a material object, a person, an event, an activity, or an abstract concept. References can take on many forms, including: a thought, a sensory perception that is Hearing (sense), audible (onomatopoeia), visual perception, visual (text), olfaction, olfactory, or tactile, emotions, emotional state, relationship with other, spacetime coordinates, symbolic system, symbolic or alpha-numeric grid, alpha-numeric, a physical object, or an energy projection. In some cases, meth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Pathology
Clinical pathology is a medical specialty that is concerned with the diagnosis of disease based on the laboratory analysis of bodily fluids, such as blood, urine, and tissue homogenates or extracts using the tools of chemistry, microbiology, hematology, molecular pathology, and Immunohaematology. This specialty requires a medical residency. Clinical pathology is a term used in the US, UK, Ireland, many Commonwealth countries, Portugal, Brazil, Italy, Japan, and Peru; countries using the equivalent in the home language of "laboratory medicine" include Austria, Germany, Romania, Poland and other Eastern European countries; other terms are "clinical analysis" (Spain) and "clinical/medical biology (France, Belgium, Netherlands, North and West Africa). Licensing and subspecialities The American Board of Pathology certifies clinical pathologists, and recognizes the following secondary specialties of clinical pathology: * Chemical pathology, also called clinical chemistry * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Joint Committees
A joint committee is a committee made up of members of the two chambers of a bicameral legislature. In other contexts, it refers to a committee with members from more than one organization. Germany A joint committee ('' Gemeinsamer Ausschuss'') comprises both members of Bundestag (two thirds) and representatives of the '' Länder'' (one third). It exists to ensure a working legislature during a state of defense. A mediation committee (''Vermittlungsausschuss''), consisting in equal numbers of members of Bundestag and representatives of the states, facilitates compromises between Bundestag and Bundesrat in legislation - especially if the consent of Bundesrat is constitutionally required. India In India, a Joint Parliamentary Committee (JPC) is one type of ad hoc Parliamentary committee constituted by the Indian parliament. A Joint Parliamentary Committee is formed when a motion is adopted by one house and it is supported or agreed by the other house. Philippines A bicameral c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |