|
International Laboratory Accreditation Cooperation
The International Laboratory Accreditation Cooperation or ILAC started as a conference in 1977 to develop international cooperation for facilitating trade by promoting the acceptance of accredited test and calibration results. In 1996, ILAC became a formal cooperation with a charter to establish a network of mutual recognition agreements among accreditation bodies that would fulfil this aim. The ultimate aim of the ILAC is increased use and acceptance by industry as well as government of the results from accredited laboratories, including results from laboratories in other countries. In this way, the free-trade goal of a 'product tested once and accepted everywhere' can be realised. See also * Accreditation * Good laboratory practice (GLP) * Institute for Reference Materials and Measurements (IRMM) * International Federation of Clinical Chemistry and Laboratory Medicine * ISO/IEC 17025 * Joint Committee for Traceability in Laboratory Medicine * Reference range * Reference ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Accreditation
Accreditation is the independent, third-party evaluation of a conformity assessment body (such as certification body, inspection body or laboratory) against recognised standards, conveying formal demonstration of its impartiality and competence to carry out specific conformity assessment tasks (such as certification, inspection and testing). Accreditation bodies are established in many economies with the primary purpose of ensuring that conformity assessment bodies are subject to oversight by an authoritative body. Accreditation bodies, that have been peer evaluated as competent, sign regional and international arrangements to demonstrate their competence. These accreditation bodies then assess and accredit conformity assessment bodies to the relevant standards. An authoritative body that performs accreditation is called an ' accreditation body'. The International Accreditation Forum (IAF) and International Laboratory Accreditation Cooperation (ILAC) provide international recogni ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Good Laboratory Practice
The Principles of Good Laboratory Practice (GLP) establish rules and criteria for a quality system that oversees the organizational processes and conditions in which non-clinical (non-pharmaceutical) health and environmental safety–or simply toxicology–studies are planned, conducted, monitored, recorded, reported, and archived. These principles apply to the toxicity testing of chemicals in commerce, to ensure the quality and integrity of the safety data submitted by manufacturers to regulatory authorities globally. History The historical events leading to the proposal of the Good Laboratory Practice (GLP) regulations are crucial for understanding why these regulations are important to improve the quality and integrity of chemical safety data. They were developed in response to concerns about the reliability of toxicity data from industry. The GLP regulations aim to standardize procedures and practices to ensure accurate, reliable, and traceable safety data. GLP was first introd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Institute For Reference Materials And Measurements
The Institute for Reference Materials and Measurements (IRMM), located in Geel, Belgium, is one of the seven institutes of the Joint Research Centre (JRC), a Directorate-General of the European Commission (EC). The IRMM promotes a common and reliable European measurement system in support of European Union policies. The institute works on the production and dissemination of quality assurance tools, such as validated methods, reference materials, reference measurements, interlaboratory comparisons and training in best practices and experience in all areas where IRMM is working. The institute was founded in 1957 under the Treaties of Rome and started operation in 1960 under the name of the Central Bureau for Nuclear Measurements (CBNM). In 1986 the programme for a Community Bureau of Reference was announced. In 1993 the institute was renamed to reflect the new mission of the institute, which covers a wide range of measurement problems from food safety to environmental pollution. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
International Federation Of Clinical Chemistry And Laboratory Medicine
The International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) is a global professional association that promotes the fields of clinical chemistry and laboratory medicine. It was established in Paris in 1952 as the International Association of Clinical Biochemists to organize the various national societies of these fields and is based in Milan, Italy. The IFCC's aims are to set global standards, support and educate its members, and provide conferences and other gatherings for sharing knowledge among the global laboratory medicine community. IFCC members fall into three groups: national societies of clinical chemistry and laboratory medicine, corporations, and affiliate international or national societies involved in laboratory medicine. As of 2023, these members represented more than 45,000 individual clinical chemists, laboratory scientists, and laboratory physicians. Structure and organization The IFCC is governed by a council consisting of representati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ISO/IEC 17025
ISO/ IEC 17025 General requirements for the competence of testing and calibration laboratories is the main standard used by testing and calibration laboratories. In most countries, ISO/IEC 17025 is the standard for which most labs must hold accreditation in order to be deemed technically competent. In many cases, suppliers and regulatory authorities will not accept test or calibration results from a lab that is not accredited. Originally known as ISO/IEC Guide 25, ISO/IEC 17025 was initially issued by ISO/IEC in 1999. There are many commonalities with the ISO 9000 standard, but ISO/IEC 17025 is more specific in requirements for competence and applies directly to those organizations that produce testing and calibration results and is based on more technical principles. Laboratories use ISO/IEC 17025 to implement a quality system aimed at improving their ability to consistently produce valid results. Material in the standard also forms the basis for accreditation from an accreditati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Joint Committee For Traceability In Laboratory Medicine
The Joint Committee for Traceability in Laboratory Medicine or JCTLM is collaboration between the International Bureau of Weights and Measures (BIPM), the International Federation for Clinical Chemistry and Laboratory Medicine (IFCC), and the International Laboratory Accreditation Cooperation (ILAC). The goal of the JCTLM is to provide a worldwide platform to promote and give guidance on internationally recognized and accepted equivalence of measurements in laboratory medicine and traceability to appropriate measurement standards. See also * Good laboratory practice (GLP) * Institute for Reference Materials and Measurements (IRMM) * Reference range * Reference values A reference is a relationship between Object (philosophy), objects in which one object designates, or acts as a means by which to connect to or link to, another object. The first object in this relation is said to ''refer to'' the second object. ... References External links Joint Committee for Traceabili ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reference Range
In medicine and health-related fields, a reference range or reference interval is the range or the interval of values that is deemed normal for a physiological measurement in healthy persons (for example, the amount of creatinine in the blood, or the partial pressure of oxygen). It is a basis for comparison for a physician or other health professional to interpret a set of test results for a particular patient. Some important reference ranges in medicine are reference ranges for blood tests and reference ranges for urine tests. The standard definition of a reference range (usually referred to if not otherwise specified) originates in what is most prevalent in a reference group taken from the general (i.e. total) population. This is the general reference range. However, there are also ''optimal health ranges'' (ranges that appear to have the optimal health impact) and ranges for particular conditions or statuses (such as pregnancy reference ranges for hormone levels). Value ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
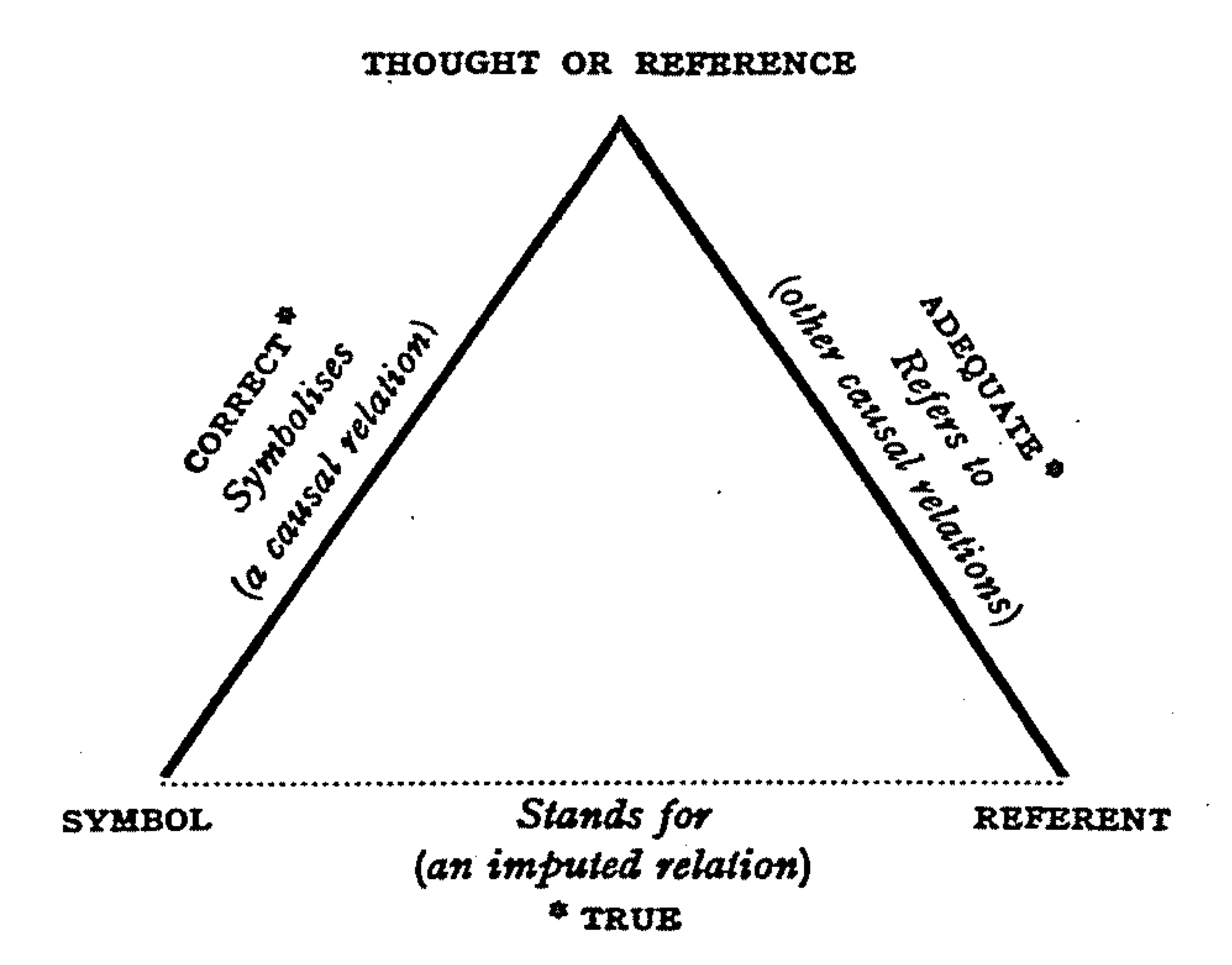
Reference Values
A reference is a relationship between Object (philosophy), objects in which one object designates, or acts as a means by which to connect to or link to, another object. The first object in this relation is said to ''refer to'' the second object. It is called a ''name'' for the second object. The next object, the one to which the first object refers, is called the ''referent'' of the first object. A name is usually a phrase or expression, or some other Symbol, symbolic representation. Its referent may be anything – a material object, a person, an event, an activity, or an abstract concept. References can take on many forms, including: a thought, a sensory perception that is Hearing (sense), audible (onomatopoeia), visual perception, visual (text), olfaction, olfactory, or tactile, emotions, emotional state, relationship with other, spacetime coordinates, symbolic system, symbolic or alpha-numeric grid, alpha-numeric, a physical object, or an energy projection. In some cases, meth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |