|
Jennifer L. Martin
Professor Jennifer Louise "Jenny" Martin is an Australian scientist and academic. She was the Deputy Vice-Chancellor (Research and Innovation) at the University of Wollongong, in New South Wales from 2019-2022. She is a former director of thGriffith Institute for Drug Discoveryat Griffith University. and a former Australian Research Council Laureate Fellow at the Institute for Molecular Bioscience, University of Queensland. Martin is Professor Emerita at the University of Queensland and Adjunct Professor at Griffith University. Her research expertise encompasses structural biology, protein crystallography, protein interactions and their applications in drug design and discovery. Education Martin completed a Bachelor of Pharmacy at the Victorian College of Pharmacy in Melbourne from 1979 to 1981, receiving the Gold Medal for the best student in the B Pharm course. After spending a year as a trainee pharmacist, she completed a Masters in Pharmacy, supervised by Professor Pete ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jenny Martin Academic Scientist
Jenny may refer to: * Jenny (given name), a popular feminine name and list of real and fictional people * Jenny (surname), a family name Animals * Jenny (donkey), a female donkey * Jenny (elephant), a female elephant in the German Army in World War I * Jenny (gorilla), the oldest gorilla in captivity at the time of her death at age 55 * Jenny (orangutan), an orangutan in the London Zoo in the 1830s Films * ''Jenny'' (1936 film), a French film by Marcel Carné * ''Jenny'' (1958 film), a Dutch film * ''Jenny'' (1962 film), an Australian television film * ''Jenny'' (1970 film), a film starring Alan Alda and Marlo Thomas Music * "Jenny" (EP), a 2003 song released as an EP single by stellastarr* * "Jenny" (The Click Five song) (2007) * "Jenny" (Nothing More song) (2015) * "Jenny" (Studio Killers song) (2013) * "867-5309/Jenny", a 1982 song by Tommy Tutone * "Jenny", a 1968 song by John Mayall & the Bluesbreakers * "Jenny", a 1973 song by Chicago from '' Chicago VI'' * "Jenny" ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
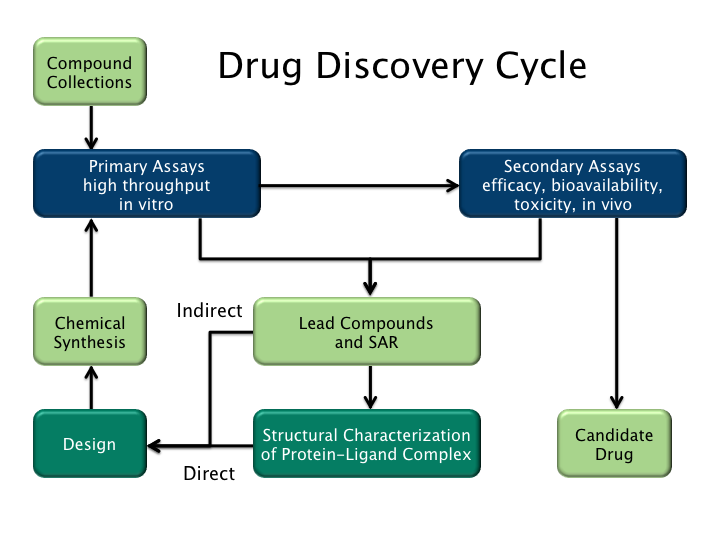
Drug Design
Drug design, often referred to as rational drug design or simply rational design, is the invention, inventive process of finding new medications based on the knowledge of a biological target. The drug is most commonly an organic compound, organic small molecule that activates or inhibits the function of a biomolecule such as a protein, which in turn results in a therapeutic effect, therapeutic benefit to the patient. In the most basic sense, drug design involves the design of molecules that are complementary in shape and electric charge, charge to the biomolecular target with which they interact and therefore will bind to it. Drug design frequently but not necessarily relies on molecular modelling, computer modeling techniques. This type of modeling is sometimes referred to as computer-aided drug design. Finally, drug design that relies on the knowledge of the three-dimensional structure of the biomolecular target is known as structure-based drug design. In addition to small molec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |