|
Isotopologue
In chemistry, isotopologues (also spelled isotopologs) are molecules that differ only in their isotopic composition. They have the same chemical formula and bonding arrangement of atoms, but at least one atom has a different number of neutrons than the parent. An example is water, whose hydrogen-related isotopologues are: "light water" (HOH or ), " semi-heavy water" with the deuterium isotope in equal proportion to protium (HDO or ), "heavy water" with two deuterium atoms ( or ); and "super-heavy water" or tritiated water ( or , as well as and , where some or all of the hydrogen is the radioactive tritium isotope). Oxygen-related isotopologues of water include the commonly available form of heavy-oxygen water () and the more difficult to separate version with the isotope. Both elements may be replaced by isotopes, for example in the doubly labeled water isotopologue . Altogether, there are 9 different stable water isotopologues, and 9 radioactive isotopologues involving tr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clumped Isotopes
Clumped isotopes are heavy isotopes that are chemical bond, bonded to other heavy isotopes. The relative abundance of clumped isotopes (and multiply-substituted Isotopologue, isotopologues) in molecules such as Methane clumped isotopes, methane, nitrous oxide, and carbonate is an area of active investigation. The carbonate clumped-isotope thermometer, or "13C–18O order/disorder carbonate thermometer", is a new approach for paleoclimate reconstruction, based on the temperature dependence of the clumping of carbon-13, 13C and oxygen-18, 18O into bonds within the carbonate crystal lattice, mineral lattice. This approach has the advantage that the 18O ratio in water is not necessary (different from the oxygen-18#Paleoclimatology, δ18O approach), but for precise paleotemperature estimation, it also needs very large and uncontaminated samples, long analytical runs, and extensive replication. Commonly used sample sources for paleoclimatological work include corals, otoliths, Gastropoda, g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopomer
Isotopomers or isotopic isomers are isomers which differ by isotopic substitution, and which have the same number of atoms of each isotope but in a different arrangement. For example, CH3OD and CH2DOH are two isotopomers of monodeuterated methanol. The molecules may be either structural isomers (constitutional isomers) or stereoisomers depending on the location of the isotopes. Isotopomers have applications in areas including nuclear magnetic resonance spectroscopy, reaction kinetics, and biochemistry. Description Isotopomers or isotopic isomers are isomers with isotopic atoms, having the same number of each isotope of each element but differing in their positions in the molecule. The result is that the molecules are either constitutional isomers or stereoisomers solely based on isotopic location. The term isotopomer was first proposed by Seeman and Paine in 1992 to distinguish isotopic isomers from isotopologues (isotopic homologues). Examples * CH3CHDCH3 and CH3CH2CH2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jeffrey I
Jeffrey may refer to: * Jeffrey (name), including a list of people with the name *Jeffrey's, Newfoundland and Labrador, Canada *Jeffrey City, Wyoming, United States *Jeffrey Street, Sydney, Australia *Jeffreys Bay, Western Cape, South Africa Art and entertainment * Jeffrey (play), ''Jeffrey'' (play), a 1992 off-Broadway play by Paul Rudnick * Jeffrey (1995 film), ''Jeffrey'' (1995 film), a 1995 film by Paul Rudnick, based on Rudnick's play of the same name *Jeffrey (2016 film), ''Jeffrey'' (2016 film), a 2016 Dominican Republic documentary film *Jeffrey's sketch, a sketch on American TV show ''Saturday Night Live'' *''Nurse Jeffrey'', a spin-off miniseries from the American medical drama series ''House, MD'' People with the surname * Alexander Jeffrey (1806–1874), Scottish solicitor and historian * Carol Jeffrey (1898–1998), English psychotherapist, writer *Charles Jeffrey (footballer) (died 1915), Scottish footballer *E. C. Jeffrey (1866–1952), Canadian-American botanist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrous Oxide
Nitrous oxide (dinitrogen oxide or dinitrogen monoxide), commonly known as laughing gas, nitrous, or factitious air, among others, is a chemical compound, an Nitrogen oxide, oxide of nitrogen with the Chemical formula, formula . At room temperature, it is a colourless Flammability#Definitions, non-flammable gas, and has a slightly sweet scent and taste. At elevated temperatures, nitrous oxide is a powerful Oxidising agent, oxidiser similar to molecular oxygen. Nitrous oxide has significant Nitrous oxide (medication), medical uses, especially in surgery and dentistry, for its Anesthesia, anaesthetic and Analgesic, pain-reducing effects, and it is on the WHO Model List of Essential Medicines, World Health Organization's List of Essential Medicines. Its colloquial name, "laughing gas", coined by Humphry Davy, describes the Euphoria, euphoric effects upon inhaling it, which cause it to be used as a recreational drug inducing a brief "Dissociative, high". When abused chronically ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. It is a common element in the universe, estimated at Abundance of the chemical elements, seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element chemical bond, bond to form N2, a colourless and odourless diatomic molecule, diatomic gas. N2 forms about 78% of Atmosphere of Earth, Earth's atmosphere, making it the most abundant chemical species in air. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth. It was first discovered and isolated by Scottish physician Daniel Rutherford in 1772 and independently by Carl Wilhelm Scheele and Henry Cavendish at about the same time. The name was suggested by French chemist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes it an economically attractive fuel, although capturing and storing it is difficult because it is a gas at standard temperature and pressure. In the Earth's atmosphere methane is transparent to visible light but absorbs infrared radiation, acting as a greenhouse gas. Methane is an Organic chemistry, organic Organic compound, compound, and among the simplest of organic compounds. Methane is also a hydrocarbon. Naturally occurring methane is found both below ground and under the seafloor and is formed by both geological and biological processes. The largest reservoir of methane is under the seafloor in the form of methane clathrates. When methane reaches the surface and the Atmosphere of Earth, atmosphere, it is known as atmospheric methane. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at normally-encountered concentrations it is odorless. As the source of carbon in the carbon cycle, atmospheric is the primary carbon source for life on Earth. In the air, carbon dioxide is transparent to visible light but absorbs infrared, infrared radiation, acting as a greenhouse gas. Carbon dioxide is soluble in water and is found in groundwater, lakes, ice caps, and seawater. It is a trace gas Carbon dioxide in Earth's atmosphere, in Earth's atmosphere at 421 parts per million (ppm), or about 0.042% (as of May 2022) having risen from pre-industrial levels of 280 ppm or about 0.028%. Burning fossil fuels is the main cause of these increased concentrations, which are the primary cause of climate change.IPCC (2022Summary for pol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paleoclimate
Paleoclimatology ( British spelling, palaeoclimatology) is the scientific study of climates predating the invention of meteorological instruments, when no direct measurement data were available. As instrumental records only span a tiny part of Earth's history, the reconstruction of ancient climate is important to understand natural variation and the evolution of the current climate. Paleoclimatology uses a variety of proxy methods from Earth and life sciences to obtain data previously preserved within rocks, sediments, boreholes, ice sheets, tree rings, corals, shells, and microfossils. Combined with techniques to date the proxies, the paleoclimate records are used to determine the past states of Earth's atmosphere. The scientific field of paleoclimatology came to maturity in the 20th century. Notable periods studied by paleoclimatologists include the frequent glaciations that Earth has undergone, rapid cooling events like the Younger Dryas, and the rapid warming durin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Equilibrium
In a chemical reaction, chemical equilibrium is the state in which both the Reagent, reactants and Product (chemistry), products are present in concentrations which have no further tendency to change with time, so that there is no observable change in the properties of the Thermodynamic system, system. This state results when the forward reaction proceeds at the same rate as the Reversible reaction, reverse reaction. The reaction rates of the forward and backward reactions are generally not zero, but they are equal. Thus, there are no net changes in the concentrations of the reactants and products. Such a state is known as dynamic equilibrium. It is the subject of study of ''equilibrium chemistry''. Historical introduction The Concept learning, concept of chemical equilibrium was developed in 1803, after Claude Louis Berthollet, Berthollet found that some chemical reactions are Reversible reaction, reversible. For any reaction mixture to exist at equilibrium, the reaction rate, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotope Geochemistry
Isotope geochemistry is an aspect of geology based upon the study of natural variations in the relative abundances of isotopes of various Chemical element, elements. Variations in isotopic abundance are measured by isotope-ratio mass spectrometry, and can reveal information about the ages and origins of rock, air or water bodies, or processes of mixing between them. Stable isotope geochemistry is largely concerned with isotopic variations arising from mass-dependent isotope fractionation, whereas radiogenic isotope geochemistry is concerned with the products of natural radioactivity. Stable isotope geochemistry For most stable isotopes, the magnitude of fractionation from kinetic fractionation, kinetic and equilibrium fractionation is very small; for this reason, enrichments are typically reported in "per mil" (Per mille, ‰, parts per thousand). These enrichments (δ) represent the ratio of heavy isotope to light isotope in the sample over the ratio of a Reference Materials fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
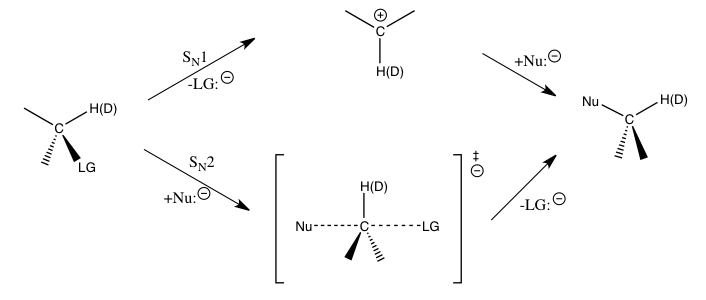
Kinetic Isotope Effect
In physical organic chemistry, a kinetic isotope effect (KIE) is the change in the reaction rate of a chemical reaction when one of the atoms in the reactants is replaced by one of its isotopes. Formally, it is the ratio of rate constants for the reactions involving the light (''k'') and the heavy (''k'') isotopically substituted reactants ( isotopologues): KIE = ''k/k''. This change in reaction rate is a quantum effect that occurs mainly because heavier isotopologues have lower vibrational frequencies than their lighter counterparts. In most cases, this implies a greater energy input needed for heavier isotopologues to reach the transition state (or, in rare cases, dissociation limit), and therefore, a slower reaction rate. The study of KIEs can help elucidate reaction mechanisms, and is occasionally exploited in drug development to improve unfavorable pharmacokinetics by protecting metabolically vulnerable C-H bonds. Background KIE is considered one of the most essential ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deuterated Chloroform
Deuterated chloroform, also known as chloroform-''d'', is the organic compound with the formula . Deuterated chloroform is a common solvent used in NMR spectroscopy. The properties of (chloroform) are virtually identical. Deuterochloroform was first made in 1935 during the years of research on deuterium. Preparation Deuterated chloroform is commercially available. It is more easily produced and less expensive than deuterated dichloromethane. Deuterochloroform is produced by the reaction of hexachloroacetone with deuterium oxide, using pyridine as a catalyst. The large difference in boiling points between the starting material and product facilitate purification by distillation. : Treating chloral with sodium deuteroxide (NaOD) gives deuterated chloroform. NMR solvent In proton NMR spectroscopy, deuterated solvent (enriched to >99% deuterium) is typically used to avoid recording a large interfering signal or signals from the proton(s) (i.e., hydrogen-1) present in the sol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |