|
Isotopes Of Selenium
Selenium has six natural isotopes that occur in significant quantities, along with the trace isotope 79Se, which occurs in minute quantities in uranium ores. Five of these isotopes are stable: 74Se, 76Se, 77Se, 78Se, and 80Se. The last three also occur as fission products, along with 79Se, which has a half-life of 327,000 years,The half-life of 79Se and 82Se, which has a very long half-life (~1020 years, decaying via to 82Kr) and for practical purposes can be considered to be stable. There are 23 other unstable isotopes that have been ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selenium
Selenium is a chemical element; it has symbol (chemistry), symbol Se and atomic number 34. It has various physical appearances, including a brick-red powder, a vitreous black solid, and a grey metallic-looking form. It seldom occurs in this elemental state or as pure ore compounds in Earth's crust. Selenium ( ) was discovered in 1817 by , who noted the similarity of the new element to the previously discovered tellurium (named for the Earth). Selenium is found in :Sulfide minerals, metal sulfide ores, where it substitutes for sulfur. Commercially, selenium is produced as a byproduct in the refining of these ores. Minerals that are pure selenide or selenate compounds are rare. The chief commercial uses for selenium today are glassmaking and pigments. Selenium is a semiconductor and is used in photocells. Applications in electronics, once important, have been mostly replaced with silicon semiconductor devices. Selenium is still used in a few types of Direct current, DC power surge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Primordial Nuclide
In geochemistry, geophysics and nuclear physics, primordial nuclides, also known as primordial isotopes, are nuclides found on Earth that have existed in their current form since before Earth was formed. Primordial nuclides were present in the interstellar medium from which the Solar System was formed, and were formed in, or after, the Big Bang, by nucleosynthesis in stars and supernovae followed by mass ejection, by cosmic ray spallation, and potentially from other processes. They are the stable nuclides plus the long-lived fraction of radionuclides surviving in the primordial solar nebula through planet accretion until the present; 286 such nuclides are known. Stability All of the known 251 stable nuclides, plus another 35 nuclides that have half-lives long enough to have survived from the formation of the Earth, occur as primordial nuclides. These 35 primordial radionuclides represent isotopes of 28 separate elements. Cadmium, tellurium, xenon, neodymium, samarium, osmi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Germanium
Germanium (32Ge) has five naturally occurring isotopes, 70Ge, 72Ge, 73Ge, 74Ge, and 76Ge. Of these, 76Ge is very slightly radioactive, decaying by double beta decay with a half-life of 1.78 × 1021 years (130 billion times the age of the universe). Stable 74Ge is the most common isotope, having a natural abundance of approximately 36%. 76Ge is the least common with a natural abundance of approximately 7%. At least 27 radioisotopes have also been synthesized ranging in atomic mass from 58 to 89. The most stable of these is 68Ge, decaying by electron capture with a half-life of 270.95 d. It decays to the medically useful positron-emitting isotope 68Ga. (See gallium-68 generator for notes on the source of this isotope, and its medical use.) The least stable known germanium isotope is 59Ge with a half-life of 13.3 ms. While most of germanium's radioisotopes decay by beta decay, 61Ge and 65Ge can also decay by β+-delayed proton emission. 84Ge through 87Ge also ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Arsenic
Arsenic (33As) has 32 known isotopes and at least 10 isomers. Only one of these isotopes, 75As, is stable; as such, it is considered a monoisotopic element. The longest-lived radioisotope is 73As with a half-life of 80 days. List of isotopes , -id=Arsenic-64 , rowspan=2 , 64As , rowspan=2 style="text-align:right" , 33 , rowspan=2 style="text-align:right" , 31 , rowspan=2 , 63.95756(22)# , rowspan=2 , 69.0(14) ms , β+ , 64Ge , rowspan=2 , 0+# , rowspan=2 , , - , β+, p? , 63Ga , -id=Arsenic-65 , rowspan=2 , 65As , rowspan=2 style="text-align:right" , 33 , rowspan=2 style="text-align:right" , 32 , rowspan=2 , 64.949611(91) , rowspan=2 , 130.3(6) ms , β+ , 65Ge , rowspan=2 , 3/2−# , rowspan=2 , , - , β+, p? , 64Ga , -id=Arsenic-66 , 66As , style="text-align:right" , 33 , style="text-align:right" , 33 , 65.9441488(61) , 95.77(23) ms , β+ , 66Ge , 0+ , , -id=Arsenic-66m1 , style="text-indent:1em" , 66m1As , colspan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Bromine
Bromine (35Br) has two stable isotopes, 79Br and 81Br, and 35 known radioisotopes, the most stable of which is 77Br, with a half-life of 57.036 hours. Like the radioactive isotopes of iodine, radioisotopes of bromine, collectively radiobromine, can be used to label biomolecules for nuclear medicine; for example, the positron emitters 75Br and 76Br can be used for positron emission tomography. Radiobromine has the advantage that organobromides are more stable than analogous organoiodides, and that it is not uptaken by the thyroid like iodine. List of isotopes , -id=Bromine-68 , 68Br , style="text-align:right" , 35 , style="text-align:right" , 33 , 67.95836(28)# , ~35 ns , p? , 67Se , 3+# , , , -id=Bromine-69 , 69Br , style="text-align:right" , 35 , style="text-align:right" , 34 , 68.950338(45) , 99.4%) , 76Br , rowspan=2, (4)+ , rowspan=2, , rowspan=2, , - , β+ (99.99%) , 78Se , rowspan=2, 1+ , rowspan=2, , rowspan=2, , - , β− (<0.0 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Krypton
There are 34 known isotopes of krypton (36Kr) with atomic mass numbers from 67 to 103. Naturally occurring krypton is made of five stable isotopes and one () which is slightly radioactive with an extremely long half-life, plus traces of radioisotopes that are produced by cosmic rays in the atmosphere. List of isotopes , -id=Krypton-67 , rowspan=2, 67Kr , rowspan=2 style="text-align:right" , 36 , rowspan=2 style="text-align:right" , 31 , rowspan=2, 66.98331(46)# , rowspan=2, 7.4(29) ms , β+? (63%) , 67Br , rowspan=2, 3/2-# , rowspan=2, , rowspan=2, , - , 2p (37%) , 65Se , -id=Krypton-68 , rowspan=3, 68Kr , rowspan=3 style="text-align:right" , 36 , rowspan=3 style="text-align:right" , 32 , rowspan=3, 67.97249(54)# , rowspan=3, 21.6(33) ms , β+, p (>90%) , 67Se , rowspan=3, 0+ , rowspan=3, , rowspan=3, , - , β+? (98.7%) , 70Br , rowspan=2, 0+ , rowspan=2, , rowspan=2, , - , β+, p (<1.3%) , 69Se , -id=Krypton-71 , rows ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Great Oxidation Event
The Great Oxidation Event (GOE) or Great Oxygenation Event, also called the Oxygen Catastrophe, Oxygen Revolution, Oxygen Crisis or Oxygen Holocaust, was a time interval during the Earth's Paleoproterozoic era when the Earth's atmosphere and shallow seas first experienced a rise in the concentration of free oxygen. This began approximately 2.460–2.426 billion years ago (Ga) during the Siderian period and ended approximately 2.060 Ga ago during the Rhyacian. Geological, isotopic and chemical evidence suggests that biologically produced molecular oxygen (dioxygen or O2) started to accumulate in the Archean prebiotic atmosphere due to microbial photosynthesis, and eventually changed it from a weakly reducing atmosphere practically devoid of oxygen into an oxidizing one containing abundant free oxygen, with oxygen levels being as high as 10% of modern atmospheric level by the end of the GOE. The appearance of highly reactive free oxygen, which can oxidize o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neoproterozoic Era
The Neoproterozoic Era is the last of the three geologic eras of the Proterozoic eon, spanning from 1 billion to 538.8 million years ago, and is the last era of the Precambrian "supereon". It is preceded by the Mesoproterozoic era and succeeded by the Paleozoic era of the Phanerozoic eon, and is further subdivided into three periods, the Tonian, Cryogenian and Ediacaran. One of the most severe glaciation events known in the geologic record occurred during the Cryogenian period of the Neoproterozoic, when global ice sheets may have reached the equator and created a "Snowball Earth" lasting about 100 million years. The earliest fossils of complex life are found in the Tonian period in the form of ''Otavia'', a primitive sponge, and the earliest fossil evidence of metazoan radiation are found in the Ediacaran period, which included the namesaked Ediacaran biota as well as the oldest definitive cnidarians and bilaterians in the fossil record. According to Rino and co-workers, t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Redox
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. The oxidation and reduction processes occur simultaneously in the chemical reaction. There are two classes of redox reactions: * Electron transfer, Electron-transfer – Only one (usually) electron flows from the atom, ion, or molecule being oxidized to the atom, ion, or molecule that is reduced. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * Atom transfer – An atom transfers from one Substrate (chemistry), substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously, the oxidation state of oxygen decreases as it accepts electrons r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iridium-192
Iridium-192 (symbol 192Ir) is a radioactive isotope of iridium, with a half-life of 73.827 days. It decays by emitting beta (β) particles and gamma (γ) radiation. About 96% of 192Ir decays occur via emission of β and γ radiation, leading to 192Pt. Some of the β particles are captured by other 192Ir nuclei, which are then converted to 192Os. Electron capture is responsible for the remaining 4% of 192Ir decays. Iridium-192 is normally produced by neutron activation of natural-abundance iridium metal. Iridium-192 is a very strong gamma ray emitter, with a gamma dose-constant of approximately 1.54 μSv·h−1· MBq−1 at 30 cm, and a specific activity of 341 TBq·g−1 (9.22 kCi·g−1). There are seven principal energy packets produced during its disintegration process ranging from just over 0.2 to about 0.6 MeV. It is commonly used as a gamma ray source in industrial radiography to locate flaws in metal components. It is also used in radiotherapy as a radiation s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
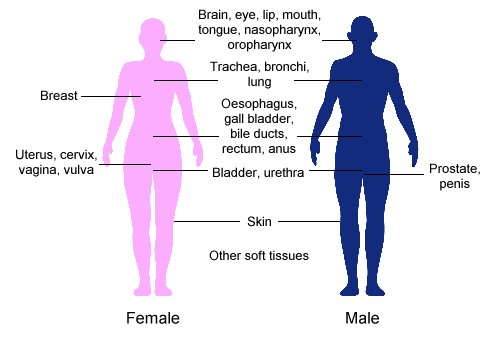
Brachytherapy
Brachytherapy is a form of radiation therapy where a sealed radiation, radiation source is placed inside or next to the area requiring treatment. The word "brachytherapy" comes from the Ancient Greek, Greek word , meaning "short-distance" or "short". Brachytherapy is commonly used as an effective treatment for cervical cancer, cervical, Prostate cancer, prostate, Breast cancer, breast, Esophageal cancer, esophageal and skin cancer and can also be used to treat tumours in many other body sites. Treatment results have demonstrated that the cancer-cure rates of brachytherapy are either comparable to surgery and external beam radiotherapy (EBRT) or are improved when used in combination with these techniques. Brachytherapy can be used alone or in combination with other therapies such as surgery, EBRT and chemotherapy. Brachytherapy contrasts with unsealed source radiotherapy, in which a therapeutic radionuclide (radioisotope) is injected into the body to chemically localize to the t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radiopharmaceutical
Radiopharmaceuticals, or medicinal radiocompounds, are a group of pharmaceutical drugs containing radioactive isotopes. Radiopharmaceuticals can be used as diagnostic and therapeutic agents. Radiopharmaceuticals emit radiation themselves, which is different from contrast media which absorb or alter external electromagnetism or ultrasound. Radiopharmacology is the branch of pharmacology that specializes in these agents. The main group of these compounds are the radiotracers used to diagnose dysfunction in body tissues. While not all medical isotopes are radioactive, radiopharmaceuticals are the oldest and remain the most common of such drugs. Drug nomenclature As with other pharmaceutical drugs, there is standardization of the drug nomenclature for radiopharmaceuticals, although various standards coexist. The International Nonproprietary Names (INNs), United States Pharmacopeia (USP) names, and IUPAC names for these agents are usually similar other than trivial style diffe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |