|
Isotopes Of Neptunium
Neptunium (93Np) is usually considered an artificial element, although trace quantities are found in nature, so a standard atomic weight cannot be given. Like all trace or artificial elements, it has no stable isotopes. The first isotope to be synthesized and identified was 239Np in 1940, produced by bombarding with neutrons to produce , which then underwent beta decay to . Trace quantities are found in nature from neutron capture reactions by uranium atoms, a fact not discovered until 1951. Twenty-five neptunium radioisotopes have been characterized, with the most stable being with a half-life of 2.14 million years, with a half-life of 154,000 years, and with a half-life of 396.1 days. All of the remaining radioactive isotopes have half-lives that are less than 4.5 days, and the majority of these have half-lives that are less than 50 minutes. This element also has five meta states, with the most stable being (t1/2 22.5 hours). The isotopes of neptunium range from to , ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neptunium
Neptunium is a chemical element; it has chemical symbol, symbol Np and atomic number 93. A radioactivity, radioactive actinide metal, neptunium is the first transuranic element. It is named after Neptune, the planet beyond Uranus in the Solar System, which uranium is named after. A neptunium atom has 93 protons and 93 electrons, of which seven are valence electrons. Neptunium metal is silvery and tarnishes when exposed to air. The element occurs in three allotrope, allotropic forms and it normally exhibits five oxidation states, ranging from +3 to +7. Like all actinides, it is Radioactive decay, radioactive, radiation poisoning, poisonous, pyrophoricity, pyrophoric, and capable of accumulating in bones, which makes the handling of neptunium dangerous. Although many false claims of its discovery were made over the years, the element was first synthesized by Edwin McMillan and Philip H. Abelson at the Berkeley Radiation Laboratory in 1940. Since then, most neptunium has been and still ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decay Product
In nuclear physics, a decay product (also known as a daughter product, daughter isotope, radio-daughter, or daughter nuclide) is the remaining nuclide left over from radioactive decay. Radioactive decay often proceeds via a sequence of steps ( decay chain). For example, 238U decays to 234Th which decays to 234mPa which decays, and so on, to 206Pb (which is stable): : \ce \overbrace^\ce left, upThe decay chain from lead-212 down to lead-208, showing the intermediate decay products In this example: * 234Th, 234mPa,...,206Pb are the decay products of 238U. * 234Th is the daughter of the parent 238U. * 234mPa (234 metastable) is the granddaughter of 238U. These might also be referred to as the daughter products of 238U. (''Depleted Uranium'' — authors: Naomi H. Harley, Ernest C. Foulkes, Lee H. Hilb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decay Energy
The decay energy is the energy change of a nucleus having undergone a radioactive decay. Radioactive decay is the process in which an unstable atomic nucleus loses energy by emitting ionizing particles and radiation. This decay, or loss of energy, results in an atom of one type (called the parent nuclide) transforming to an atom of a different type (called the daughter nuclide). Decay calculation The energy difference of the reactants is often written as ''Q'': :Q = \left( \text \right)_\text - \left( \text \right)_\text, :Q = \left(\text \right)_ c^2 - \left( \text \right )_\text c^2 . Decay energy is usually quoted in terms of the energy units MeV (million electronvolts) or keV (thousand electronvolts): : Q \text = -931.5 \Delta M \text,~~(\text\Delta M = \Sigma M_\text - \Sigma M_\text). Types of radioactive decay include * gamma ray * beta decay (decay energy is divided between the emitted electron and the neutrino which is emitted at the same time) * alpha decay Alp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isomeric Transition
A nuclear isomer is a metastable state of an atomic nucleus, in which one or more nucleons (protons or neutrons) occupy excited state levels (higher energy levels). "Metastable" describes nuclei whose excited states have half-lives of 10−9 seconds or longer, 100 to 1000 times longer than the half-lives of the excited nuclear states that decay with a "prompt" half life (ordinarily on the order of 10−12 seconds). Some references recommend seconds to distinguish the metastable half life from the normal "prompt" gamma-emission half-life. Occasionally the half-lives are far longer than this and can last minutes, hours, or years. For example, the nuclear isomer survives so long (at least years) that it has never been observed to decay spontaneously. The half-life of a nuclear isomer can even exceed that of the ground state of the same nuclide, as shown by as well as , , , , and multiple holmium isomers. Sometimes, the gamma decay from a metastable state is referred ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
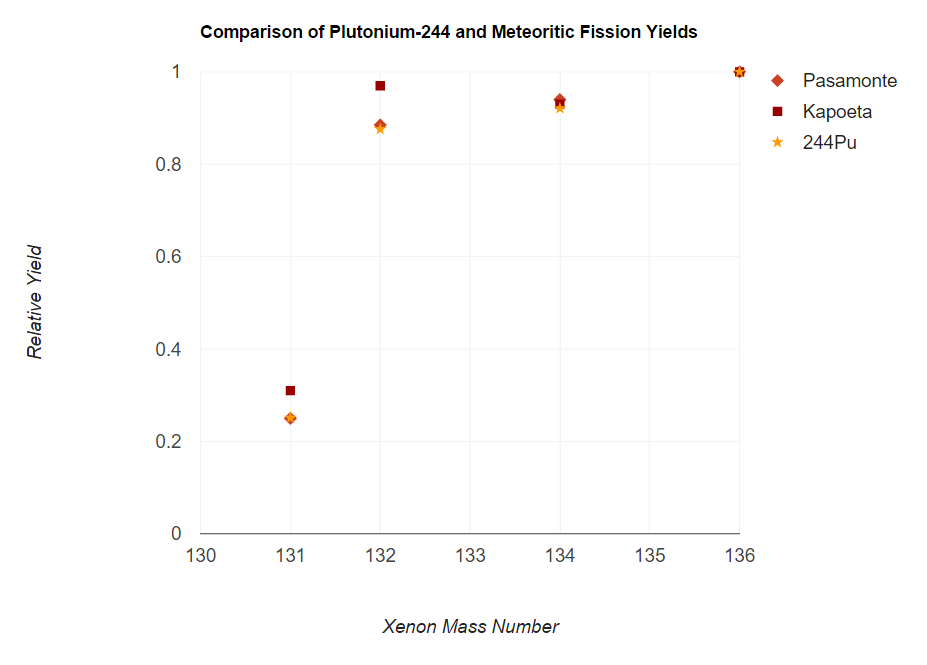
Plutonium-244
Plutonium-244 (Pu) is an isotope of plutonium that has a half-life of 81.3 million years. This is longer than any other isotope of plutonium and longer than any other known isotope of an element beyond bismuth, except for the three naturally abundant ones: uranium-235 (704 million years), uranium-238 (4.468 billion years), and thorium-232 (14.05 billion years). Given the half-life of Pu, an exceedingly small amount should still be present on Earth, making plutonium a likely but unproven candidate as the shortest-lived primordial element. Natural occurrence Accurate measurements, beginning in the early 1970s, appeared to detect primordial plutonium-244, making it the shortest-lived primordial nuclide. The amount of Pu in the pre-Solar nebula (4.57×10 years ago) was estimated as 0.8% the amount of U. As the age of the Earth is about 56 half-lives of Pu, the amount of Pu left should be very small; Hoffman et al. estimated its content in the rare-earth mineral bastnasite as & ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cluster Decay
Cluster decay, also named heavy particle radioactivity, heavy ion radioactivity or heavy cluster decay," is a rare type of nuclear decay in which an atomic nucleus emits a small "cluster" of neutrons and protons, more than in an alpha particle, but less than a typical binary fission fragment. Ternary fission into three fragments also produces products in the cluster size. Description The loss of protons from the parent nucleus changes it to the nucleus of a different element, the daughter, with a mass number ''Ad'' = ''A'' − ''Ae'' and atomic number ''Zd'' = ''Z'' − ''Ze'', where ''Ae'' = ''Ne'' + ''Ze''. For example: : → + According to "Ronen's golden rule" of cluster decay, the emitted nucleus tends to be one with a high binding energy per nucleon, and especially one with a magic number of nucleons. This type of rare decay mode was observed in radioisotopes that decay predominantly by alpha emission, and it occurs only in a small percentage of the decays for al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fissile
In nuclear engineering, fissile material is material that can undergo nuclear fission when struck by a neutron of low energy. A self-sustaining thermal Nuclear chain reaction#Fission chain reaction, chain reaction can only be achieved with fissile material. The predominant Neutron temperature, neutron energy in a system may be typified by either slow neutrons (i.e., a thermal system) or fast neutrons. Fissile material can be used to fuel thermal-neutron reactors, fast-neutron reactors and nuclear explosives. Fissile vs fissionable The term ''fissile'' is distinct from ''fissionable''. A nuclide that can undergo nuclear fission (even with a low probability) after capturing a neutron of high or low energy is referred to as ''fissionable''. A fissionable nuclide that can undergo fission with a high probability after capturing a low-energy thermal neutron is referred to as ''fissile''. Fissionable materials include those (such as uranium-238) for which fission can be induce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Capture
Electron capture (K-electron capture, also K-capture, or L-electron capture, L-capture) is a process in which the proton-rich nucleus of an electrically neutral atom absorbs an inner atomic electron, usually from the K or L electron shells. This process thereby changes a nuclear proton to a neutron and simultaneously causes the emission of an electron neutrino. : : or when written as a nuclear reaction equation, ^_e + ^_p -> ^_n + ^_ ν_e Since this single emitted neutrino carries the entire decay energy, it has this single characteristic energy. Similarly, the momentum of the neutrino emission causes the daughter atom to recoil with a single characteristic momentum. The resulting daughter nuclide, if it is in an excited state, then transitions to its ground state. Usually, a gamma ray is emitted during this transition, but nuclear de-excitation may also take place by internal conversion. Following capture of an inner electron from the atom, an outer elect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spontaneous Fission
Spontaneous fission (SF) is a form of radioactive decay in which a heavy atomic nucleus splits into two or more lighter nuclei. In contrast to induced fission, there is no inciting particle to trigger the decay; it is a purely probabilistic process. Spontaneous fission is a dominant decay mode for superheavy elements, with nuclear stability generally falling as nuclear mass increases. It thus forms a practical limit to heavy element nucleon number. Heavier nuclides may be created instantaneously by physical processes, both natural (via the r-process, ''r''-process) and artificial, though rapidly decay to more stable nuclides. As such, apart from minor decay branches in primordial radionuclides, spontaneous fission is not observed in nature. Observed fission half-lives range from 60 nanoseconds () to greater than the current age of the universe (). History Following the discovery of induced fission by Otto Hahn and Fritz Strassmann in 1938, Soviet physicists Georgy Flyorov and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha Decay
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus). The parent nucleus transforms or "decays" into a daughter product, with a mass number that is reduced by four and an atomic number that is reduced by two. An alpha particle is identical to the nucleus of a helium-4 atom, which consists of two protons and two neutrons. It has a charge of and a mass of , and is represented as ^_\alpha. For example, uranium-238 undergoes alpha decay to form thorium-234. While alpha particles have a charge , this is not usually shown because a nuclear equation describes a nuclear reaction without considering the electrons – a convention that does not imply that the nuclei necessarily occur in neutral atoms. Alpha decay typically occurs in the heaviest nuclides. Theoretically, it can occur only in nuclei somewhat heavier than nickel (element 28), where the overall binding energy per nucleon is no longer a maximum a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Physical Review Letters
''Physical Review Letters'' (''PRL''), established in 1958, is a peer-reviewed, scientific journal that is published 52 times per year by the American Physical Society. The journal is considered one of the most prestigious in the field of physics. Over a quarter of Physics Nobel Prize-winning papers between 1995 and 2017 were published in it. ''PRL'' is published both online and as a print journal. Its focus is on short articles ("letters") intended for quick publication. The Lead Editor is Hugues Chaté. The Managing Editor is Robert Garisto. History The journal was created in 1958. Samuel Goudsmit, who was then the editor of '' Physical Review'', the American Physical Society's flagship journal, organized and published ''Letters to the Editor of Physical Review'' into a new standalone journal'','' which became ''Physical Review Letters''. It was the first journal intended for the rapid publication of short articles, a format that eventually became popular in many other fiel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proton Drip Line
The nuclear drip line is the boundary beyond which atomic nuclei are unbound with respect to the emission of a proton or neutron. An arbitrary combination of protons and neutrons does not necessarily yield a stable nucleus. One can think of moving up or to the right across the table of nuclides by adding a proton or a neutron, respectively, to a given nucleus. However, adding nucleons one at a time to a given nucleus will eventually lead to a newly formed nucleus that immediately decays by emitting a proton (or neutron). Colloquially speaking, the nucleon has ''leaked'' or ''dripped'' out of the nucleus, hence giving rise to the term ''drip line''. Drip lines are defined for protons and neutrons at the extreme of the proton-to-neutron ratio; at p:n ratios at or beyond the drip lines, no bound nuclei can exist. While the location of the proton drip line is well known for many elements, the location of the neutron drip line is only known for elements up to neon. General descr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |