 |
Isothermal Process
An isothermal process is a type of thermodynamic process in which the temperature ''T'' of a system remains constant: Δ''T'' = 0. This typically occurs when a system is in contact with an outside thermal reservoir, and a change in the system occurs slowly enough to allow the system to be continuously adjusted to the temperature of the reservoir through heat exchange (see quasi-equilibrium). In contrast, an '' adiabatic process'' is where a system exchanges no heat with its surroundings (''Q'' = 0). Simply, we can say that in an isothermal process * T = \text * \Delta T = 0 * dT = 0 * For ideal gases only, internal energy \Delta U = 0 while in adiabatic processes: * Q = 0. Etymology The noun '' isotherm'' is derived from the Ancient Greek words (), meaning "equal", and (), meaning "heat". Examples Isothermal processes can occur in any kind of system that has some means of regulating the temperature, including highly structured machines, and even living ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
|
Thermodynamic System
A thermodynamic system is a body of matter and/or radiation separate from its surroundings that can be studied using the laws of thermodynamics. Thermodynamic systems can be passive and active according to internal processes. According to internal processes, passive systems and active systems are distinguished: passive, in which there is a redistribution of available energy, active, in which one type of energy is converted into another. Depending on its interaction with the environment, a thermodynamic system may be an isolated system, a Closed system#In thermodynamics, closed system, or an Open system (systems theory), open system. An isolated system does not exchange matter or energy with its surroundings. A closed system may exchange heat, experience forces, and exert forces, but does not exchange matter. An open system can interact with its surroundings by exchanging both matter and energy. The physical condition of a thermodynamic system at a given time is described by its ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
|
|
Phase Changes
In physics, chemistry, and other related fields like biology, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic states of matter: solid, liquid, and gas, and in rare cases, plasma. A phase of a thermodynamic system and the states of matter have uniform physical properties. During a phase transition of a given medium, certain properties of the medium change as a result of the change of external conditions, such as temperature or pressure. This can be a discontinuous change; for example, a liquid may become gas upon heating to its boiling point, resulting in an abrupt change in volume. The identification of the external conditions at which a transformation occurs defines the phase transition point. Types of phase transition States of matter Phase transitions commonly refer to when a substance transforms between one of the four states of matter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
|
 |
James Watt
James Watt (; 30 January 1736 (19 January 1736 OS) – 25 August 1819) was a Scottish inventor, mechanical engineer, and chemist who improved on Thomas Newcomen's 1712 Newcomen steam engine with his Watt steam engine in 1776, which was fundamental to the changes brought by the Industrial Revolution in both his native Great Britain and the rest of the world. While working as an instrument maker at the University of Glasgow, Watt became interested in the technology of steam engines. At the time engineers such as John Smeaton were aware of the inefficiencies of Newcomen's engine and aimed to improve it. Watt's insight was to realise that contemporary engine designs wasted a great deal of energy by repeatedly cooling and reheating the cylinder. Watt introduced a design enhancement, the separate condenser, which avoided this waste of energy and radically improved the power, efficiency, and cost-effectiveness of steam engines. Eventually, he adapted his engine to produce rot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
|
Indicator Diagram
An indicator diagram is a chart used to measure the thermal, or cylinder, performance of Reciprocating engine, reciprocating steam and Internal combustion engine, internal combustion engines and compressors. An indicator chart records the pressure in the cylinder versus the volume swept by the piston, throughout the two or four strokes of the piston which constitute the engine, or compressor, cycle. The indicator diagram is used to calculate the work (thermodynamics), work done and the power produced in an engine cylinder or used in a compressor cylinder. The indicator diagram was developed by James Watt and his employee John Southern (engineer), John Southern to help understand how to improve the Energy conversion efficiency, efficiency of steam engines. In 1796, Southern developed the simple, but critical, technique to generate the diagram by fixing a board so as to move with the piston, thereby tracing the "volume" axis, while a pencil, attached to a pressure gauge, moved at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
|
 |
Ideal Gas Law
The ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. It is a good approximation of the behavior of many gases under many conditions, although it has several limitations. It was first stated by Benoît Paul Émile Clapeyron in 1834 as a combination of the empirical Boyle's law, Charles's law, Avogadro's law, and Gay-Lussac's law. The ideal gas law is often written in an empirical form: pV = nRT where p, V and T are the pressure, volume and Thermodynamic temperature, temperature respectively; n is the amount of substance; and R is the ideal gas constant. It can also be derived from the microscopic kinetic theory of gases, kinetic theory, as was achieved (independently) by August Krönig in 1856 and Rudolf Clausius in 1857. Equation The state function, state of an amount of gas is determined by its pressure, volume, and temperature. The modern form of the equation relates these simply in two main forms. The temperature us ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
|
Ideal Gas Constant
The molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol or . It is the molar equivalent to the Boltzmann constant, expressed in units of energy per temperature increment per amount of substance, rather than energy per temperature increment per ''particle''. The constant is also a combination of the constants from Boyle's law, Charles's law, Avogadro's law, and Gay-Lussac's law. It is a physical constant that is featured in many fundamental equations in the physical sciences, such as the ideal gas law, the Arrhenius equation, and the Nernst equation. The gas constant is the constant of proportionality that relates the energy scale in physics to the temperature scale and the scale used for amount of substance. Thus, the value of the gas constant ultimately derives from historical decisions and accidents in the setting of units of energy, temperature and amount of substance. The Boltzmann constant and the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
|
 |
Mole (unit)
The mole (symbol mol) is a unit of measurement, the base unit in the International System of Units (SI) for ''amount of substance'', an SI base quantity proportional to the number of elementary entities of a substance. One mole is an aggregate of exactly elementary entities (approximately 602 sextillion or 602 billion times a trillion), which can be atoms, molecules, ions, ion pairs, or other particles. The number of particles in a mole is the Avogadro number (symbol ) and the numerical value of the '' Avogadro constant'' (symbol ) expressed in mol−1. The relationship between the mole, Avogadro number, and Avogadro constant can be expressed in the following equation:1\text = \frac = \frac The current SI value of the mole is based on the historical definition of the mole as the amount of substance that corresponds to the number of atoms in 12 grams of 12C, which made the molar mass of a compound in grams per mole, numerically equal to the average molecular mass or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
 |
Boyle's Law
Boyle's law, also referred to as the Boyle–Mariotte law or Mariotte's law (especially in France), is an empirical gas laws, gas law that describes the relationship between pressure and volume of a confined gas. Boyle's law has been stated as: The absolute pressure exerted by a given mass of an ideal gas is inversely proportional to the volume it occupies if the temperature and amount of substance, amount of gas remain unchanged within a closed system.Levine (1978) p. 12 gives the original definition. Mathematically, Boyle's law can be stated as: or where is the pressure of the gas, is the volume of the gas, and is a Constant (mathematics), constant for a particular temperature and amount of gas. Boyle's law states that when the temperature of a given mass of confined gas is constant, the product of its pressure and volume is also constant. When comparing the same substance under two different sets of conditions, the law can be expressed as: P_1 V_1 = P_2 V_2. showi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
|
Ideal Gas Isotherms
Ideal may refer to: Philosophy * Ideal (ethics), values that one actively pursues as goals * Platonic ideal, a philosophical idea of trueness of form, associated with Plato Mathematics * Ideal (ring theory), special subsets of a ring considered in abstract algebra * Ideal, special subsets of a semigroup * Ideal (order theory), special kind of lower sets of an order * Ideal (set theory), a collection of sets regarded as "small" or "negligible" * Ideal (Lie algebra), a particular subset in a Lie algebra * Ideal point, a boundary point in hyperbolic geometry * Ideal triangle, a triangle in hyperbolic geometry whose vertices are ideal points Science * Ideal chain, in science, the simplest model describing a polymer * Ideal gas law, in physics, governing the pressure of an ideal gas * Ideal transformer, an electrical transformer having zero resistance and perfect magnetic threading * Ideal final result, in TRIZ methodology, the best possible solution * Thought experiment, somet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
|
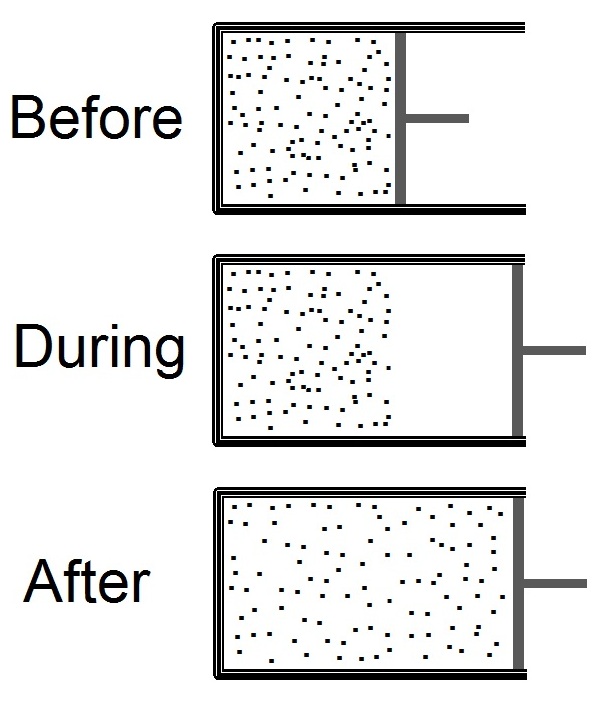 |
Joule Expansion
The Joule expansion (a subset of free expansion) is an irreversible process in thermodynamics in which a volume of gas is kept in one side of a thermally isolated container (via a small partition), with the other side of the container being evacuated. The partition between the two parts of the container is then opened, and the gas fills the whole container. The Joule expansion, treated as a thought experiment involving ideal gases, is a useful exercise in classical thermodynamics. It provides a convenient example for calculating changes in thermodynamic quantities, including the resulting increase in entropy of the universe (entropy production) that results from this inherently irreversible process. An actual Joule expansion experiment necessarily involves real gases; the temperature change in such a process provides a measure of intermolecular forces. This type of expansion is named after James Prescott Joule who used this expansion, in 1845, in his study for the mechanical e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
|
Isothermal Process
An isothermal process is a type of thermodynamic process in which the temperature ''T'' of a system remains constant: Δ''T'' = 0. This typically occurs when a system is in contact with an outside thermal reservoir, and a change in the system occurs slowly enough to allow the system to be continuously adjusted to the temperature of the reservoir through heat exchange (see quasi-equilibrium). In contrast, an '' adiabatic process'' is where a system exchanges no heat with its surroundings (''Q'' = 0). Simply, we can say that in an isothermal process * T = \text * \Delta T = 0 * dT = 0 * For ideal gases only, internal energy \Delta U = 0 while in adiabatic processes: * Q = 0. Etymology The noun '' isotherm'' is derived from the Ancient Greek words (), meaning "equal", and (), meaning "heat". Examples Isothermal processes can occur in any kind of system that has some means of regulating the temperature, including highly structured machines, and even living ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
|
|
Intermolecular Forces
An intermolecular force (IMF; also secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction or repulsion which act between atoms and other types of neighbouring particles (e.g. atoms or ions). Intermolecular forces are weak relative to intramolecular forces – the forces which hold a molecule together. For example, the covalent bond, involving sharing electron pairs between atoms, is much stronger than the forces present between neighboring molecules. Both sets of forces are essential parts of force fields frequently used in molecular mechanics. The first reference to the nature of microscopic forces is found in Alexis Clairaut's work ''Théorie de la figure de la Terre,'' published in Paris in 1743. Other scientists who have contributed to the investigation of microscopic forces include: Laplace, Gauss, Maxwell, Boltzmann and Pauling. Attractive intermolecular forces are categorized into the following type ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |