|
Isoconazole
Isoconazole is an azole antifungal drug and could inhibit gram positive bacteria. For foot and vaginal infections, isoconazole has a similar effectiveness to clotrimazole. Isoconazole nitrate may be used in combination with corticosteroid diflucortolone to increase its bioavailability In pharmacology, bioavailability is a subcategory of absorption and is the fraction (%) of an administered drug that reaches the systemic circulation. By definition, when a medication is administered intravenously, its bioavailability is 100%. H .... It was patented in 1968 and approved for medical use in 1979. References 21-Hydroxylase inhibitors Aromatase inhibitors Chloroarenes CYP17A1 inhibitors Phenylethanolamine ethers Imidazole antifungals Lanosterol 14α-demethylase inhibitors {{genito-urinary-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antifungal Drug
An antifungal medication, also known as an antimycotic medication, is a pharmaceutical fungicide or fungistatic used to treat and prevent mycosis such as athlete's foot, ringworm, candidiasis (thrush), serious systemic infections such as cryptococcal meningitis, and others. Such drugs are usually obtained by a doctor's prescription, but a few are available over the counter (OTC). The evolution of antifungal resistance is a growing threat to health globally. Routes of administration Ocular Indicated when the fungal infection is located in the eye. There is currently only one ocular antifungal available: natamycin. However, various other antifungal agents could be compounded in this formulation. Intrathecal Used occasionally when there's an infection of the central nervous system and other systemic options cannot reach the concentration required in that region for therapeutic benefit. Example(s): amphotericin B. Vaginal This may be used to treat some fungal inf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azole
Azoles are a class of five-membered heterocyclic compounds containing a nitrogen atom and at least one other non-carbon atom (i.e. nitrogen, sulfur, or oxygen) as part of the ring. Their names originate from the Hantzsch–Widman nomenclature. The parent compounds are aromatic and have two double bonds; there are successively redox, reduced analogs (azolines and azolidines) with fewer. One, and only one, lone pair of electrons from each heteroatom in the ring is part of the aromatic bonding in an azole. Names of azoles maintain the prefix upon reduction (e.g., pyrazoline, pyrazolidine). The numbering of ring atoms in azoles starts with the heteroatom that is not part of a double bond, and then proceeds towards the other heteroatom. Imidazole and other five-membered aromatic heterocyclic systems with two nitrogens are extremely common in nature and form the core of many biomolecules, such as histidine. Compound classes ;Nitrogen only Imidazol.svg, Imidazole Pyrazol.svg, Pyrazol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
The Merck Index
''The Merck Index'' is an encyclopedia of chemicals, drugs and biologicals with over 10,000 monographs on single substances or groups of related compounds published online by the Royal Society of Chemistry. History The first edition of the Merck's Index was published in 1889 by the German chemical company Emanuel Merck and was primarily used as a sales catalog for Merck's growing list of chemicals it sold. The American subsidiary was established two years later and continued to publish it. During World War I the US government seized Merck's US operations and made it a separate American "Merck" company that continued to publish the Merck Index. In 2012 the Merck Index was licensed to the Royal Society of Chemistry. An online version of The Merck Index, including historic records and new updates not in the print edition, is commonly available through research libraries. It also includes an appendix with monographs on organic named reactions. The 15th edition was published in A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
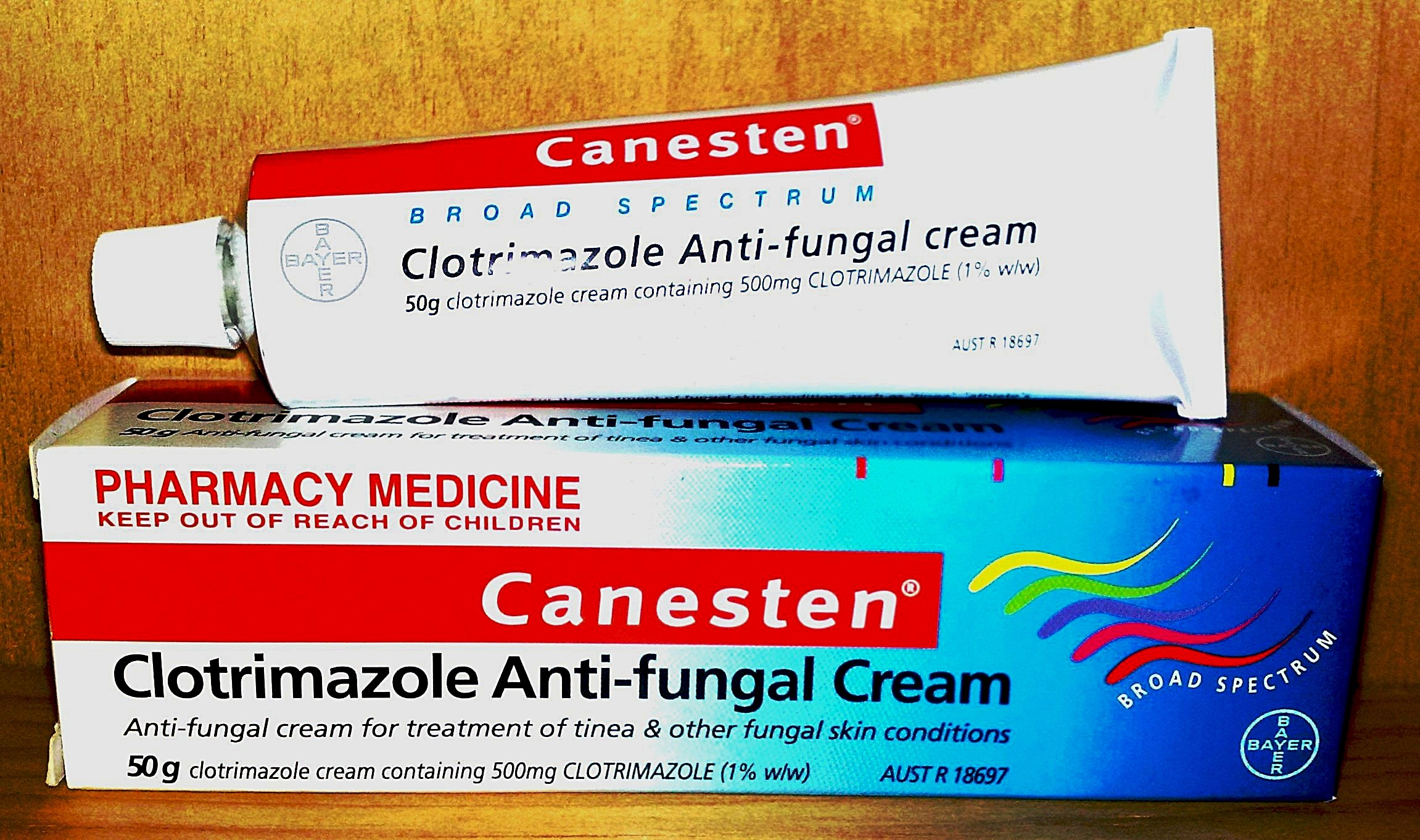
Clotrimazole
Clotrimazole, sold under the brand name Lotrimin, among others, is an antifungal medication. It is used to treat vaginal yeast infections, oral thrush, diaper rash, tinea versicolor, and types of ringworm including athlete's foot and jock itch. It can be taken by mouth or applied as a cream to the skin or in the vagina. Common side effects when taken by mouth include nausea and itchiness. When applied to the skin, common side effects include redness and a burning sensation. In pregnancy, use on the skin or in the vagina is believed to be safe. There is no evidence of harm when used by mouth during pregnancy but this has been less well studied. When used by mouth, greater care should be taken in those with liver problems. It is in the azole class of medications and works by disrupting the fungal cell membrane. Clotrimazole was discovered in 1969. It is on the WHO Model List of Essential Medicines, World Health Organization's List of Essential Medicines. It is available as a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Corticosteroid
Corticosteroids are a class of steroid hormones that are produced in the adrenal cortex of vertebrates, as well as the synthetic analogues of these hormones. Two main classes of corticosteroids, glucocorticoids and mineralocorticoids, are involved in a wide range of physiological processes, including stress response, immune response, and regulation of inflammation, carbohydrate metabolism, protein catabolism, blood electrolyte levels, and behavior. Some common naturally occurring steroid hormones are cortisol (), corticosterone (), cortisone () and aldosterone () (cortisone and aldosterone are isomers). The main corticosteroids produced by the adrenal cortex are cortisol and aldosterone. The etymology of the '' cortico-'' part of the name refers to the adrenal cortex, which makes these steroid hormones. Thus a corticosteroid is a "cortex steroid". Classes * Glucocorticoids such as cortisol affect carbohydrate, fat, and protein metabolism, and have anti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diflucortolone
Diflucortolone (INN), or difluocortolone, is a corticosteroid Corticosteroids are a class of steroid hormones that are produced in the adrenal cortex of vertebrates, as well as the synthetic analogues of these hormones. Two main classes of corticosteroids, glucocorticoids and mineralocorticoids, are invo .... See also * Diflucortolone valerate References Organofluorides {{dermatologic-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bioavailability
In pharmacology, bioavailability is a subcategory of absorption and is the fraction (%) of an administered drug that reaches the systemic circulation. By definition, when a medication is administered intravenously, its bioavailability is 100%. However, when a medication is administered via routes other than intravenous, its bioavailability is lower due to intestinal epithelium absorption and first-pass metabolism. Thereby, mathematically, bioavailability equals the ratio of comparing the area under the plasma drug concentration curve versus time (AUC) for the extravascular formulation to the AUC for the intravascular formulation. AUC is used because AUC is proportional to the dose that has entered the systemic circulation. Bioavailability of a drug is an average value; to take population variability into account, deviation range is shown as ±. To ensure that the drug taker who has poor absorption is dosed appropriately, the bottom value of the deviation range is employed ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aromatase Inhibitors
Aromatase inhibitors (AIs) are a class of medication, drugs used in the treatment of breast cancer in menopause, postmenopausal women and in men, and gynecomastia in men. They may also be used off-label to reduce estrogen conversion when supplementing testosterone exogenously. They may also be used for chemoprevention in women at high risk for breast cancer. Aromatase is the enzyme that catalyzes a key aromatization step in the synthesis of estrogen. It converts the Alpha-beta Unsaturated carbonyl compounds, enone ring of androgen precursors such as testosterone, to a phenol, completing the synthesis of estrogen. As such, AIs are estrogen synthesis inhibitors. Because hormone-positive breast and ovarian cancers are dependent on estrogen for growth, AIs are taken to either block the production of estrogen or block the action of estrogen on receptors. Medical uses Cancer In contrast to premenopausal women, in whom most of the estrogen is produced in the ovary, ovaries, in postmeno ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloroarenes
In organic chemistry, an aryl halide (also known as a haloarene) is an aromatic compound in which one or more hydrogen atoms directly bonded to an aromatic ring are replaced by a halide ion (such as fluorine F''−'', chlorine Cl−1,−3,−5, bromine Br−1, or iodine I−). Aryl halides are distinct from haloalkanes (alkyl halides) due to significant differences in their methods of preparation, chemical reactivity, and physical properties. The most common and important members of this class are aryl chlorides, but the group encompasses a wide range of derivatives with diverse applications in organic synthesis, pharmaceuticals, and materials science. Classification according to halide Aryl fluorides Aryl fluorides are used as synthetic intermediates, e.g. for the preparation of pharmaceuticals, pesticides, and liquid crystals. The conversion of diazonium salts is a well established route to aryl fluorides. Thus, anilines are precursors to aryl fluorides. In the classic Schiemann ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CYP17A1 Inhibitors
A CYP17A1 inhibitor is a type of drug that enzyme inhibitor, inhibits the enzyme CYP17A1. CYP17A1 inhibitors work by blocking specific enzyme functions, impacting androgen biosynthesis. Mechanism of action CYP17A1 inhibitors may inhibit one or both of the enzyme’s functions: 17α-hydroxylase and 17,20-lyase. Some inhibitors are selective and target only the 17,20-lyase function, while others inhibit both functions. By inhibiting these enzymatic functions, CYP17A1 inhibitors prevent the conversion of pregnane steroids into androgens like testosterone. This action classifies them as androgen biosynthesis inhibitors and functional antiandrogens. Examples Examples of CYP17A1 inhibitors include the older drug ketoconazole and the newer drugs abiraterone acetate, orteronel, galeterone, and seviteronel. Clinical uses CYP17A1 inhibitors, such as abiraterone acetate, are primarily used in the treatment of prostate cancer. These drugs reduce androgen levels, which helps to slow ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenylethanolamine Ethers
Phenylethanolamine (sometimes abbreviated PEOH), or β-hydroxyphenethylamine, is a trace amine with a structure similar to those of other trace phenethylamines as well as the catecholamine neurotransmitters dopamine, norepinephrine, and epinephrine. As an organic compound, phenylethanolamine is a β-hydroxylated phenethylamine that is also structurally related to a number of synthetic drugs in the substituted phenethylamine class. In common with these compounds, phenylethanolamine has strong cardiovascular activity and, under the name ''Apophedrin'', has been used as a drug to produce topical vasoconstriction.''The Merck Index, 10th Ed.'' (1983), p. 1051, Merck & Co., Rahway. In appearance, phenylethanolamine is a white solid. Phenylethanolamine is perhaps best known in the field of bioscience as part of the enzyme name " phenylethanolamine N-methyl transferase", referring to an enzyme which is responsible for the conversion of norepinephrine into epinephrine, as well as other r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |