|
Iron(II) Sulfide
Iron(II) sulfide or ferrous sulfide (Br.E. sulphide) is one of a family of chemical compounds and minerals with the approximate chemical formula, formula . Iron sulfides are often iron-deficient non-stoichiometric. All are black, water-insoluble solids. Preparation and structure FeS can be obtained by the heating of iron and sulfur: :Fe + S → FeS FeS adopts the nickel arsenide structure, featuring octahedral molecular geometry, octahedral Fe centers and trigonal prismatic sulfide sites. Reactions Iron sulfide reacts with hydrochloric acid, releasing hydrogen sulfide: :FeS + 2 HCl → FeCl2 + H2S :FeS + H2SO4 → FeSO4 + H2S In moist air, iron sulfides oxidize to hydrated ferrous sulfate. Biology and biogeochemistry Iron sulfides occur widely in nature in the form of iron–sulfur proteins. As organic matter decays under low-oxygen (or Hypoxia (environmental), hypoxic) conditions such as in swamps or Dead zone (ecology), dead zones of lakes and oceans, sul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acid
An acid is a molecule or ion capable of either donating a proton (i.e. Hydron, hydrogen cation, H+), known as a Brønsted–Lowry acid–base theory, Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis acid. The first category of acids are the proton donors, or Brønsted–Lowry acid–base theory, Brønsted–Lowry acids. In the special case of aqueous solutions, proton donors form the hydronium ion H3O+ and are known as Acid–base reaction#Arrhenius theory, Arrhenius acids. Johannes Nicolaus Brønsted, Brønsted and Martin Lowry, Lowry generalized the Arrhenius theory to include non-aqueous solvents. A Brønsted–Lowry or Arrhenius acid usually contains a hydrogen atom bonded to a chemical structure that is still energetically favorable after loss of H+. Aqueous Arrhenius acids have characteristic properties that provide a practical description of an acid. Acids form aqueous solutions with a sour taste, can turn blue litmus red, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Octahedral Molecular Geometry
In chemistry, octahedral molecular geometry, also called square bipyramidal, describes the shape of compounds with six atoms or groups of atoms or ligands symmetrically arranged around a central atom, defining the vertices of an octahedron. The octahedron has eight faces, hence the prefix '' octa''. The octahedron is one of the Platonic solids, although octahedral molecules typically have an atom in their centre and no bonds between the ligand atoms. A perfect octahedron belongs to the point group Oh. Examples of octahedral compounds are sulfur hexafluoride SF6 and molybdenum hexacarbonyl Mo(CO)6. The term "octahedral" is used somewhat loosely by chemists, focusing on the geometry of the bonds to the central atom and not considering differences among the ligands themselves. For example, , which is not octahedral in the mathematical sense due to the orientation of the bonds, is referred to as octahedral. The concept of octahedral coordination geometry was developed by Alfred ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Harold McGee
Harold James McGee (born October 3, 1951) is an American author who writes about the chemistry and history of food science and cooking. He is best known for his seminal book '' On Food and Cooking: The Science and Lore of the Kitchen'', first published in 1984'' On Food and Cooking: The Science and Lore of the Kitchen'' (1984) and revised in 2004. Early life McGee was born on 3 October 1951 in Cambridge, Massachusetts, to Louise (Hanney) and Charles Gilbert McGee, and raised in Elmhurst, Illinois. He was educated at the California Institute of Technology (Caltech), initially studying astronomy, but graduating with a B.S. in Literature in 1973. He went on to do a Ph.D. on the romantic poetry of John Keats supervised by Harold Bloom at Yale University, graduating in 1978. Career Before becoming a food science writer, McGee was a literature and writing instructor at Yale. He has also written for ''Nature'', ''Health'', ''The New York Times'', the ''World Book Encyclopedia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
McGee On Food And Cooking
''On Food And Cooking: The Science And Lore Of The Kitchen'' is a book by Harold McGee, published by Scribner in the United States in 1984 and revised extensively for a 2004 second edition. It is published by Hodder & Stoughton in Britain under the title ''McGee on Food and Cooking: An Encyclopedia of Kitchen Science, History and Culture''. The book provides a reference to the scientific understanding and preparation of food. It has been described by Alton Brown as "the Rosetta stone of the culinary world", Daniel Boulud has called the book a "must for every cook who possesses an inquiring mind", while Heston Blumenthal has stated it is "the book that has had the greatest single impact on my cooking".Hirst, Christopher, ''The Independent''. (November 13, 2004Snail porridge? It's a matter of taste/ref> The work is separated into sections that focus on the ingredients, providing the structure for the author to speculate on the history of foodstuffs and cookery, and the molec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Yolk
Among animals which produce eggs, the yolk (; also known as the vitellus) is the nutrient-bearing portion of the egg whose primary function is to supply food for the development of the embryo. Some types of egg contain no yolk, for example because they are laid in situations where the food supply is sufficient (such as in the body of the host (biology), host of a parasitoid) or because the embryo develops in the parent's body, which supplies the food, usually through a placenta. Reproductive systems in which the mother's body supplies the embryo directly are said to be matrotrophy, matrotrophic; those in which the embryo is supplied by yolk are said to be lecithotrophy, lecithotrophic. In many species, such as all birds, and most reptiles and insects, the yolk takes the form of a special storage organ constructed in the reproductive system, reproductive tract of the mother. In many other animals, especially very small species such as some fish and invertebrates, the yolk mate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Desulfovibrionales
Desulfovibrionales are a taxonomic order of bacteria belonging to the phylum Thermodesulfobacteriota, with four families. They are Gram-negative. The majority are sulfate-reducing, with the exception of '' Lawsonia'' and '' Bilophila''.Garrity, George M.; Brenner, Don J.; Krieg, Noel R.; Staley, James T. (eds.) (2005). Bergey's Manual of Systematic Bacteriology, Volume Two: The Proteobacteria, Part C: The Alpha-, Beta-, Delta-, and Epsilonproteobacteria. New York, New York: Springer. . All members of this order are obligately anaerobic. Most species are mesophilic, but some are moderate thermophiles. Phylogeny The currently accepted taxonomy is based on the List of Prokaryotic names with Standing in Nomenclature (LPSN) and National Center for Biotechnology Information (NCBI) See also * List of bacterial orders * List of bacteria genera This article lists the genera of the bacteria Bacteria (; : bacterium) are ubiquitous, mostly free-living organisms often con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrrhotite
Pyrrhotite (''Pyrrhus of Epirus, pyrrhos'' in Greek language, Greek meaning "flame-coloured"'')'' is an iron sulfide mineral with the formula Fe(1−x)S (x = 0 to 0.125). It is a nonstoichiometric compound, nonstoichiometric variant of FeS, the mineral known as troilite. Pyrrhotite is also called magnetic pyrite, because the color is similar to pyrite and it is weakly magnetic. The magnetism decreases as the iron content increases, and troilite is non-magnetic. Pyrrhotite is generally tabular and brassy/bronze in color with a Lustre (mineralogy), metallic luster. The mineral occurs with Mafic, mafic igneous rocks like Norite, norites, and may form from pyrite during Metamorphism, metamorphic processes. Pyrrhotite is associated and mined with other sulfide minerals like pentlandite, pyrite, chalcopyrite, and magnetite, and has been found globally. Structure Pyrrhotite exists as a number of polytypes of Hexagonal crystal system, hexagonal or monoclinic crystal symmetry; sever ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfate
The sulfate or sulphate ion is a polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many are prepared from that acid. Spelling "Sulfate" is the spelling recommended by IUPAC, but "sulphate" was traditionally used in British English. Structure The sulfate anion consists of a central sulfur atom surrounded by four equivalent oxygen atoms in a tetrahedral arrangement. The symmetry of the isolated anion is the same as that of methane. The sulfur atom is in the +6 oxidation state while the four oxygen atoms are each in the −2 state. The sulfate ion carries an overall charge of −2 and it is the conjugate base of the bisulfate (or hydrogensulfate) ion, , which is in turn the conjugate base of , sulfuric acid. Organic sulfate esters, such as dimethyl sulfate, are covalent compounds and esters of sulfuric acid. The tetrahedral ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfate-reducing Bacteria
Sulfate-reducing microorganisms (SRM) or sulfate-reducing prokaryotes (SRP) are a group composed of sulfate-reducing bacteria (SRB) and sulfate-reducing archaea (SRA), both of which can perform anaerobic respiration utilizing sulfate () as terminal electron acceptor, reducing it to hydrogen sulfide (H2S). Therefore, these sulfidogenic microorganisms "breathe" sulfate rather than Allotropes of oxygen, molecular oxygen (O2), which is the terminal electron acceptor reduced to water (H2O) in Anaerobic respiration, aerobic respiration. Most sulfate-reducing microorganisms can also reduce some other oxidized inorganic sulfur Chemical compound, compounds, such as sulfite (), dithionite (), thiosulfate (), trithionate (), tetrathionate (), Allotropes of sulfur, elemental sulfur (S8), and polysulfides (). Other than sulfate reduction, some sulfate-reducing microorganisms are also capable of other reactions like disproportionation of sulfur compounds. Depending on the context, "sulfate-reduc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dead Zone (ecology)
Dead zones are hypoxic (low-oxygen) areas in the world's oceans and large lakes. Hypoxia occurs when dissolved oxygen (DO) concentration falls to or below 2 ml of O2/liter. When a body of water experiences hypoxic conditions, aquatic flora and fauna begin to change behavior in order to reach sections of water with higher oxygen levels. Once DO declines below 0.5 ml O2/liter in a body of water, mass mortality occurs. With such a low concentration of DO, these bodies of water fail to support the aquatic life living there. Historically, many of these sites were naturally occurring. However, in the 1970s, oceanographers began noting increased instances and expanses of dead zones. These occur near inhabited coastlines, where aquatic life is most concentrated. Coastal regions, such as the Baltic Sea, the northern Gulf of Mexico, and the Chesapeake Bay, as well as large enclosed water bodies like Lake Erie, have been affected by deoxygenation due to eutrophication. Excess nutr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hypoxia (environmental)
Hypoxia (''hypo'': 'below', ''oxia'': 'oxygenated') refers to low oxygen conditions. Hypoxia is problematic for air-breathing organisms, yet it is essential for many anaerobic organisms. Hypoxia applies to many situations, but usually refers to the atmosphere and natural waters. Atmospheric hypoxia Atmospheric hypoxia occurs naturally at high altitudes. Total atmospheric pressure decreases as altitude increases, causing a lower partial pressure of oxygen, which is defined as hypobaric hypoxia. Oxygen remains at 20.9% of the total gas mixture, differing from hypoxic hypoxia, where the percentage of oxygen in the air (or blood) is decreased. This is common in the sealed burrows of some subterranean animals, such as blesmols. Atmospheric hypoxia is also the basis of altitude training, which is a standard part of training for elite athletes. Several companies mimic hypoxia using normobaric artificial atmosphere. Aquatic hypoxia An aquatic system lacking dissolved oxygen (0% ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
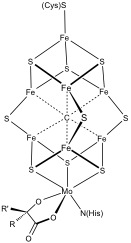
Iron–sulfur Protein
Iron–sulfur proteins are proteins characterized by the presence of iron–sulfur clusters containing sulfide-linked di-, tri-, and tetrairon centers in variable oxidation states. Iron–sulfur clusters are found in a variety of metalloproteins, such as the ferredoxins, as well as NADH dehydrogenase, hydrogenases, coenzyme Q – cytochrome c reductase, succinate – coenzyme Q reductase and nitrogenase. Iron–sulfur clusters are best known for their role in the oxidation-reduction reactions of electron transport in mitochondria and chloroplasts. Both Complex I and Complex II of oxidative phosphorylation have multiple Fe–S clusters. They have many other functions including catalysis as illustrated by aconitase, generation of radicals as illustrated by SAM-dependent enzymes, and as sulfur donors in the biosynthesis of lipoic acid and biotin. Additionally, some Fe–S proteins regulate gene expression. Fe–S proteins are vulnerable to attack by biogenic nitric oxide, formin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |