|
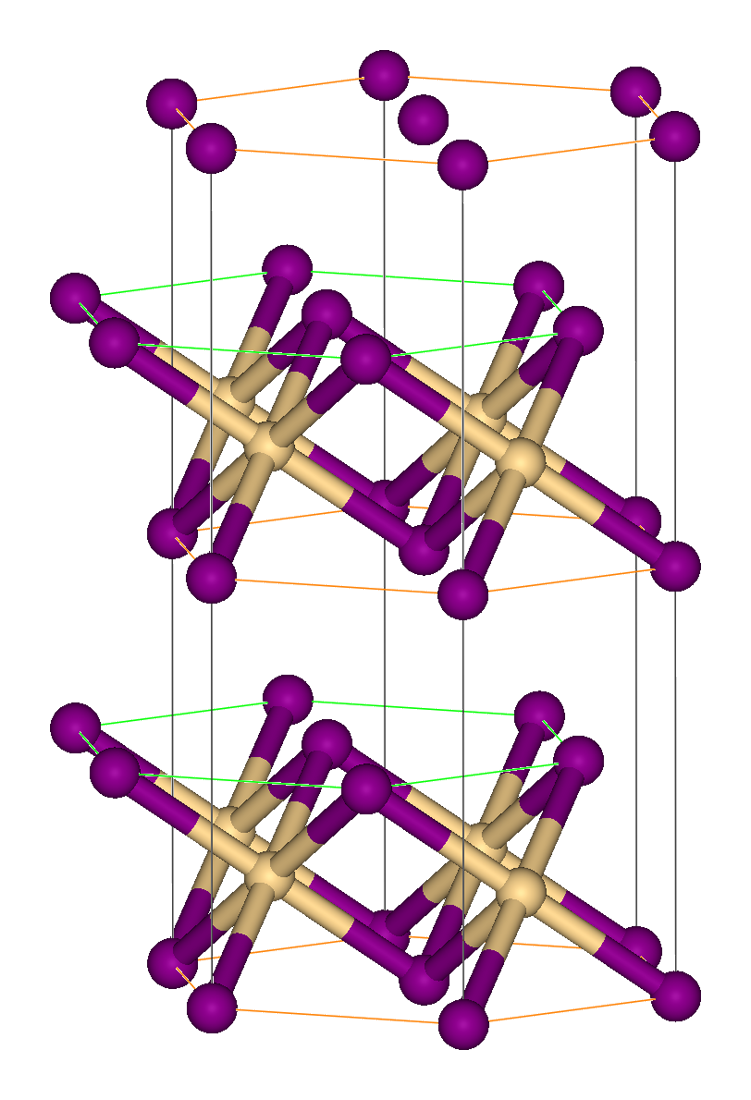
Iron(II) Iodide
Iron(II) iodide is an inorganic compound with the chemical formula FeI2. It is used as a catalyst in organic reactions. Preparation Iron(II) iodide can be synthesised from the elements, i.e. by the reaction of with . :Fe + I2 → FeI2 This is in contrast to the other iron(II) halides, which are best prepared by reaction of heated iron with the appropriate |
Iron(II) Fluoride
Iron(II) fluoride or ferrous fluoride is an inorganic compound with the molecular formula FeF2. It forms a tetrahydrate FeF2·4H2O that is often referred to by the same names. The anhydrous and hydrated forms are white crystalline solids.Dale L. Perry (1995),Handbook of Inorganic Compounds, page 167. CRC Press. Structure and bonding Anhydrous FeF2 adopts the TiO2 rutile structure. As such, the iron cations are octahedral and fluoride anions are trigonal planar. The tetrahydrate can exist in two structures, or polymorphs. One form is rhombohedral and the other is hexagonal, the former having a disorder. Like most fluoride compounds, the anhydrous and hydrated forms of iron(II) fluoride feature high spin metal center. Low temperature neutron diffraction studies show that the FeF2 is antiferromagnetic. Heat capacity measurements reveal an event at 78.3 K corresponding to ordering of antiferromagnetic state. Selected physical properties FeF2 sublimes between 958 and 1178 K. Us ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in front of oxygen (32.1% and 30.1%, respectively), forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust. In its metallic state, iron is rare in the Earth's crust, limited mainly to deposition by meteorites. Iron ores, by contrast, are among the most abundant in the Earth's crust, although extracting usable metal from them requires kilns or furnaces capable of reaching or higher, about higher than that required to smelt copper. Humans started to master that process in Eurasia during the 2nd millennium BCE and the use of iron tools and weapons began to displace copper alloys, in some regions, only around 1200 BCE. That event is considered the transition from the Bronze Age to the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron(II) Compounds
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in front of oxygen (32.1% and 30.1%, respectively), forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust. In its metallic state, iron is rare in the Earth's crust, limited mainly to deposition by meteorites. Iron ores, by contrast, are among the most abundant in the Earth's crust, although extracting usable metal from them requires kilns or furnaces capable of reaching or higher, about higher than that required to smelt copper. Humans started to master that process in Eurasia during the 2nd millennium BCE and the use of iron tools and weapons began to displace copper alloys, in some regions, only around 1200 BCE. That event is considered the transition from the Bronze Age to the Iron Age. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroiodic Acid
Hydroiodic acid (or hydriodic acid) is an aqueous solution of hydrogen iodide (HI). It is a strong acid, one that is ionized completely in an aqueous solution. It is colorless. Concentrated solutions are usually 48% to 57% HI. Reactions Hydroiodic acid reacts with oxygen in air to give iodine: :4 HI + O2 → 2 + 2 I2 Like other hydrogen halides, hydroiodic acid adds to alkenes to give alkyl iodides. It can also be used as a reducing agent, for example in the reduction of aromatic nitro compounds to anilines. Cativa process The Cativa process is a major end use of hydroiodic acid, which serves as a co-catalyst for the production of acetic acid by the carbonylation of methanol. Illicit uses Hydroiodic acid is listed as a U.S. Federal DEA List I Chemical, owing to its use as a reducing agent related to the production of methamphetamine from ephedrine or pseudoephedrine Pseudoephedrine (PSE) is a sympathomimetic drug of the phenethylamine and amphetamine chemical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cadmium Iodide
Cadmium iodide is the inorganic compound with the formula CdI2. It is a white hygroscopic solid. It also can be obtained as a mono- and tetrahydrate. It has few applications. It is notable for its crystal structure, which is typical for compounds of the form MX2 with strong polarization effects. Preparation Cadmium iodide is prepared by the addition of cadmium metal, or its oxide, hydroxide or carbonate to hydroiodic acid. Also, the compound can be made by heating cadmium with iodine. Crystal structure In cadmium iodide the iodide anions form a hexagonal close packed arrangement while the cadmium cations fill all of the octahedral sites in alternate layers. The resultant structure consists of a layered lattice. This same basic structure is found in many other salts and minerals. Cadmium iodide is mostly ionically bonded but with partial covalent character. Cadmium iodide's crystal structure is the prototype on which the crystal structures many other compounds can be conside ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystal Structure
In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions or molecules in a crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric patterns that repeat along the principal directions of three-dimensional space in matter. The smallest group of particles in the material that constitutes this repeating pattern is the unit cell of the structure. The unit cell completely reflects the symmetry and structure of the entire crystal, which is built up by repetitive translation of the unit cell along its principal axes. The translation vectors define the nodes of the Bravais lattice. The lengths of the principal axes, or edges, of the unit cell and the angles between them are the lattice constants, also called ''lattice parameters'' or ''cell parameters''. The symmetry properties of the crystal are described by the concept of space groups. All possible symmetric arrangements of partic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrohalic Acid
In chemistry, hydrogen halides (hydrohalic acids when in the aqueous phase) are diatomic, inorganic compounds that function as Arrhenius acids. The formula is HX where X is one of the halogens: fluorine, chlorine, bromine, iodine, or astatine. All known hydrogen halides are gases at Standard Temperature and Pressure.The Acidity of the Hydrogen Halides. (2020, August 21). Retrieved May 5, 2021, from https://chem.libretexts.org/@go/page/3699 Vs. hydrohalic acids The hydrogen halides are diatomic molecules with no tendency to ionize in the gas phase (although liquified hydrogen fluoride is a polar solvent somewhat similar to water). Thus, chemists distinguish hydrogen chloride from hydrochloric acid. The former is a gas at room temperature that reacts with water to give the acid. Once the acid has formed, the diatomic molecule can be regenerated only with difficulty, but not by normal distillation. Commonly the names of the acid and the molecules are not clearly distinguish ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodine
Iodine is a chemical element with the Symbol (chemistry), symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , and boils to a violet gas at . The element was discovered by the French chemist Bernard Courtois in 1811 and was named two years later by Joseph Louis Gay-Lussac, after the Ancient Greek 'violet-coloured'. Iodine occurs in many oxidation states, including iodide (I−), iodate (), and the various periodate anions. It is the least abundant of the stable halogens, being the sixty-first most abundant element. As the heaviest essential mineral nutrient, iodine is required for the synthesis of thyroid hormones. Iodine deficiency affects about two billion people and is the leading preventable cause of intellectual disabilities. The dominant producers of iodine today are Chile and Japan. Due to its high atomic number and ease of attachment to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and ''plus'' (+) and ''minus'' (−) signs. These are limited to a single typographic line of symbols, which may include subscripts and superscripts. A chemical formula is not a chemical name, and it contains no words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulae can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than chemical names and structural formulae. The simplest types of chemical formulae are called '' empirical formulae'', which use letters and numbers indicating the numerical ''proportions'' of ato ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron(II) Chloride
Iron(II) chloride, also known as ferrous chloride, is the chemical compound of formula FeCl2. It is a paramagnetic solid with a high melting point. The compound is white, but typical samples are often off-white. FeCl2 crystallizes from water as the greenish tetrahydrate, which is the form that is most commonly encountered in commerce and the laboratory. There is also a dihydrate. The compound is highly soluble in water, giving pale green solutions. Production Hydrated forms of ferrous chloride are generated by treatment of wastes from steel production with hydrochloric acid. Such solutions are designated "spent acid," or "pickle liquor" especially when the hydrochloric acid is not completely consumed: :Fe + 2 HCl → FeCl2 + H2 The spent acid requires treatment if it is disposed. Ferrous chloride is used in the manufacturing of ferric chloride. Ferrous chloride can also be used to regenerate hydrochloric acid. It is also a byproduct from titanium production, since ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Compound
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemistry''. Inorganic compounds comprise most of the Earth's crust, although the compositions of the deep mantle remain active areas of investigation. Some simple carbon compounds are often considered inorganic. Examples include the allotropes of carbon ( graphite, diamond, buckminsterfullerene, etc.), carbon monoxide, carbon dioxide, carbides, and the following salts of inorganic anions: carbonates, cyanides, cyanates, and thiocyanates. Many of these are normal parts of mostly organic systems, including organisms; describing a chemical as inorganic does not necessarily mean that it does not occur within living things. History Friedrich Wöhler's conversion of ammonium cyanate into urea in 1828 is often cited as the starting p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron(III) Iodide
Iron(III) iodide is an inorganic compound with the chemical formula FeI3. It is a thermodynamically unstable compound that is difficult to prepare. Nevertheless, iron(III) iodide has been synthesised in small quantities in the absence of air and water. Preparation Iron(III) and iodide tend to undergo a redox reaction in which Fe3+ is reduced to Fe2+ and I− is oxidised to I2. This reaction can be avoided and iron(III) iodide can be synthesised by a photochemical reaction. Iron pentacarbonyl reacts with excess iodine in hexane under argon, releasing carbon monoxide and forming the complex diiodotetracarbonyliron(II), Fe(CO)4I2, as a light red solution. :Fe(CO)5 + I2 → Fe(CO)4I2 + CO This complex then undergoes oxidative photodecarbonylation at −20 °C in the presence of further iodine and actinic light. A black film of FeI3 is deposited as further carbon monoxide is evolved. :Fe(CO)4I2 + ½I2 + ''hν'' → FeI3 + 4CO Reactivity Iron(III) iodide is prone to light-in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.png)