|
Iridium Tribromide
Iridium(III) bromide is a bromide of iridium(III), with the chemical formula of IrBr3. Preparation Iridium(III) bromide can be formed by reacting iridium(II) bromide and bromine. Its tetrahydrate can be formed by reacting iridium dioxide dihydrate with hydrobromic acid. It can also be formed by the direct reaction of iridium and bromine at 8 atm and 570 °C. Properties Iridium(III) bromide is a dark reddish-brown solid that is insoluble soluble in water, acids, and alkalis and decomposes to iridium(II) bromide on heating. It crystallizes in a highly disordered layered structure of aluminum(III) chloride or chromium(III) chloride type, where the monoclinic unit cell contains four formula units. As with rhenium(III) chloride, rhenium(III) bromide, α-iridium(III) chloride and α-ruthenium(III) chloride, the disorder is due to the different stacking of the metal layers. The light olive green tetrahydrate is slightly soluble in water but insoluble in ethanol and eth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iridium(II) Bromide
Iridium is a chemical element; it has the Symbol (chemistry), symbol Ir and atomic number 77. This very hard, brittle, silvery-white transition metal of the platinum group, is considered the second-densest naturally occurring metal (after osmium) with a density of as defined by experimental X-ray crystallography. 191Ir and 193Ir are the only two naturally occurring isotopes of iridium, as well as the only stable isotopes; the latter is the more abundant. It is one of the most corrosion-resistant metals, even at temperatures as high as . Iridium was discovered in 1803 in the acid-insoluble residues of platinum ores by the English chemist Smithson Tennant. The name ''iridium'', derived from the Greek word ''iris'' (rainbow), refers to the various colors of its compounds. Iridium is Abundance of elements in Earth's crust, one of the rarest elements in Crust (geology)#Earth's crust, Earth's crust, with an estimated annual production of only in 2023. The dominant uses of iridium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromium(III) Chloride
Chromium(III) chloride (also called chromic chloride) is an inorganic chemical compound with the chemical formula . This crystalline salt forms several hydrates with the formula , among which are hydrates where ''n'' can be 5 (chromium(III) chloride pentahydrate ) or 6 (chromium(III) chloride hexahydrate ). The anhydrous compound with the formula are violet crystals, while the most common form of the chromium(III) chloride are the dark green crystals of hexahydrate, . Chromium chlorides find use as catalysts and as precursors to dyes for wool. Structure Anhydrous chromium(III) chloride adopts the structure, with occupying one third of the octahedral interstices in alternating layers of a pseudo- cubic close packed lattice of ions. The absence of cations in alternate layers leads to weak bonding between adjacent layers. For this reason, crystals of cleave easily along the planes between layers, which results in the flaky (micaceous) appearance of samples of chromium(III) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Germanium Dibromide
Germanium dibromide is a bromide of germanium with the chemical formula GeBr2. Preparation Germanium dibromide can be obtained by reducing germanium tetrabromide with germanium or zinc.Georg Brauer (Hrsg.), unter Mitarbeit von Marianne Baudler u. a.: ''Handbuch der Präparativen Anorganischen Chemie.'' 3., umgearbeitete Auflage. Band I, Ferdinand Enke, Stuttgart 1975, ISBN 3-432-02328-6, S. 724. : Properties Germanium dibromide is a yellow-white solid that is soluble in ethanol and acetone. It disproportionates into germanium tetrabromide and germanium. It hydrolyzes to germanium dihydroxide. Germanium dibromide is monoclinic, space group P21/c (No. 14), lattice parameters a = 11.68 Å, b = 9.12 Å, c = 7.02 Å, and β = 101.9°. It can react with cyclopentadienylsodium or cyclopentadienylthallium in ether In organic chemistry, ethers are a class of compounds that contain an ether group, a single oxygen atom bonded to two separate carbon atoms, each part of an o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a solvent). It is vital for all known forms of life, despite not providing food energy or organic micronutrients. Its chemical formula, , indicates that each of its molecules contains one oxygen and two hydrogen atoms, connected by covalent bonds. The hydrogen atoms are attached to the oxygen atom at an angle of 104.45°. In liquid form, is also called "water" at standard temperature and pressure. Because Earth's environment is relatively close to water's triple point, water exists on Earth as a solid, a liquid, and a gas. It forms precipitation in the form of rain and aerosols in the form of fog. Clouds consist of suspended droplets of water and ice, its solid state. When finely divided, crystalline ice ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ether
In organic chemistry, ethers are a class of compounds that contain an ether group, a single oxygen atom bonded to two separate carbon atoms, each part of an organyl group (e.g., alkyl or aryl). They have the general formula , where R and R′ represent the organyl groups. Ethers can again be classified into two varieties: if the organyl groups are the same on both sides of the oxygen atom, then it is a simple or symmetrical ether, whereas if they are different, the ethers are called mixed or unsymmetrical ethers. A typical example of the first group is the solvent and anaesthetic diethyl ether, commonly referred to simply as "ether" (). Ethers are common in organic chemistry and even more prevalent in biochemistry, as they are common linkages in carbohydrates and lignin. Structure and bonding Ethers feature bent linkages. In dimethyl ether, the bond angle is 111° and C–O distances are 141 pm. The barrier to rotation about the C–O bonds is low. The bonding of ox ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the pseudoelement symbol for ethyl group, ethyl. Ethanol is a Volatility (chemistry), volatile, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. As a psychoactive depressant, it is the active ingredient in alcoholic beverages, and the second most consumed drug globally behind caffeine. Ethanol is naturally produced by the fermentation process of sugars by yeasts or via petrochemical processes such as ethylene hydration. Historically it was used as a general anesthetic, and has modern medical applications as an antiseptic, disinfectant, solvent for some medications, and antidote for methanol poisoning and ethylene glycol poisoning. It is used as a chemical solvent and in the Chemical synthesis, synthesis of orga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ruthenium(III) Chloride
Ruthenium(III) chloride is the chemical compound with the formula RuCl3. "Ruthenium(III) chloride" more commonly refers to the hydrate RuCl3·''x''H2O. Both the anhydrous and hydrated species are dark brown or black solids. The hydrate, with a varying proportion of water of crystallization, often approximating to a trihydrate, is a commonly used starting material in ruthenium chemistry. Preparation and properties Anhydrous ruthenium(III) chloride is usually prepared by heating powdered ruthenium metal with chlorine. In the original synthesis, the chlorination was conducted in the presence of carbon monoxide, the product being carried by the gas stream and crystallising upon cooling. Two polymorphs of RuCl3 are known. The black α-form adopts the CrCl3-type structure with long Ru-Ru contacts of 346 pm. This polymorph has honeycomb layers of Ru3+ which are surrounded with an octahedral cage of Cl− anions. The ruthenium cations are magnetic residing in a low-spin J~1/2 ground stat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iridium(III) Chloride
Iridium(III) chloride is the inorganic compound with the formula IrCl3. The anhydrous compound is relatively rare, but the related hydrate is much more commonly encountered. The anhydrous salt has two polymorphs, α and β, which are brown and red colored respectively. More commonly encountered is the hygroscopic dark green trihydrate IrCl3(H2O)3 which is a common starting point for iridium chemistry. Preparation Iridium is separated from the other platinum group metals as crystalline ammonium hexachloroiridate, (NH4)2 rCl6 which can be reduced to iridium metal in a stream of hydrogen. The spongy Ir thus produced reacts with chlorine at 650 °C to give iridium(III) chloride. Hydrated iridium trichloride is obtained by heating hydrated iridium(III) oxide with hydrochloric acid. Structure Like the related rhodium compound, IrCl3 adopts the structure seen for aluminium chloride. This is the monoclinic α polymorph. A rhombohedral β polymorph also exists. Both polymorphs h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rhenium(III) Bromide
Rhenium(III) bromide is a chemical compound with the formula Re3Br9. It is a black lustrous crystalline solid. This compound reacts with water to form rhenium(IV) oxide and is isostructural with rhenium(III) chloride. Preparation This compound is prepared by the reaction of rhenium metal and bromine gas at 500 °C under nitrogen: :6Re + 9Br2 → 2Re3Br9 If there is oxygen in the atmosphere, it will instead form rhenium(III) oxybromide. However, the most common method of producing this compound is by first reacting potassium hexabromorhenate(IV) with silver nitrate Silver nitrate is an inorganic compound with chemical formula . It is a versatile precursor to many other silver compounds, such as those used in photography. It is far less sensitive to light than the halides. It was once called ''lunar causti ..., which forms silver hexabromorhenite(IV), then this compound is heated to 600 °C to form rhenium(III) bromide. :K2ReBr6 + 2AgNO3 → Ag2ReBr6 + 2KNO3 :6Ag2ReBr6 → 12AgB ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rhenium(III) Chloride
Trirhenium nonachloride is a compound with the formula ReCl3, sometimes also written Re3Cl9. It is a dark red hygroscopic solid that is insoluble in ordinary solvents. The compound is important in the history of inorganic chemistry as an early example of a cluster compound with metal-metal bonds. It is used as a starting material for synthesis of other rhenium complexes. Structure and physical properties As shown by X-ray crystallography trirhenium nonachloride consists of Re3Cl12 subunits that share three chloride bridges with adjacent clusters. The interconnected network of clusters forms sheets. Around each Re center are seven ligands, four bridging chlorides, one terminal chloride, and two Re-Re bonds.Colton, R. Chemistry of rhenium and technetium. 965. The hydrate is molecular with the formula Re3Cl9(H2O)3. The heat of oxidation is evaluated according to the equation: :1/3 Re3Cl9 + 4 OH− + 2 OCl− → ReO4− + 2 H2O + 5Cl− The enthalpy for this process is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Formula Unit
In chemistry, a formula unit is the smallest unit of a non-molecular substance, such as an ionic compound, covalent network solid, or metal. It can also refer to the chemical formula for that unit. Those structures do not consist of discrete molecules, and so for them, the term formula unit is used. In contrast, the terms molecule or molecular formula are applied to molecules. The formula unit is used as an independent entity for stoichiometric calculations. Examples of formula units, include ionic compounds such as and and covalent networks such as and C (as diamond or graphite).Steven S. Zumdahl; Susan A. Zumdahl (2000), ''Chemistry'' (5 ed.), Houghton Mifflin, pp. 470-6, In most cases the formula representing a formula unit will also be an empirical formula, such as calcium carbonate () or sodium chloride (), but it is not always the case. For example, the ionic compounds potassium persulfate (), mercury(I) nitrate , and sodium peroxide , have empirical formulas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
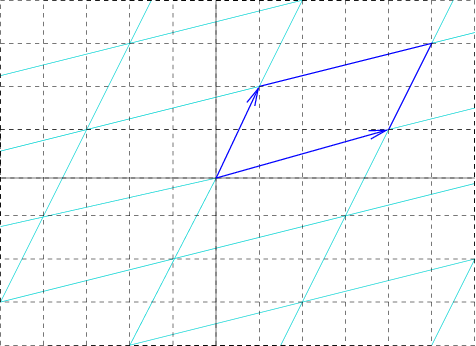
Unit Cell
In geometry, biology, mineralogy and solid state physics, a unit cell is a repeating unit formed by the vectors spanning the points of a lattice. Despite its suggestive name, the unit cell (unlike a unit vector In mathematics, a unit vector in a normed vector space is a Vector (mathematics and physics), vector (often a vector (geometry), spatial vector) of Norm (mathematics), length 1. A unit vector is often denoted by a lowercase letter with a circumfle ..., for example) does not necessarily have unit size, or even a particular size at all. Rather, the primitive cell is the closest analogy to a unit vector, since it has a determined size for a given lattice and is the basic building block from which larger cells are constructed. The concept is used particularly in describing crystal structure in two and three dimensions, though it makes sense in all dimensions. A lattice can be characterized by the geometry of its unit cell, which is a section of the tiling (a parallelogra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |