|
Ion-selective Electrodes
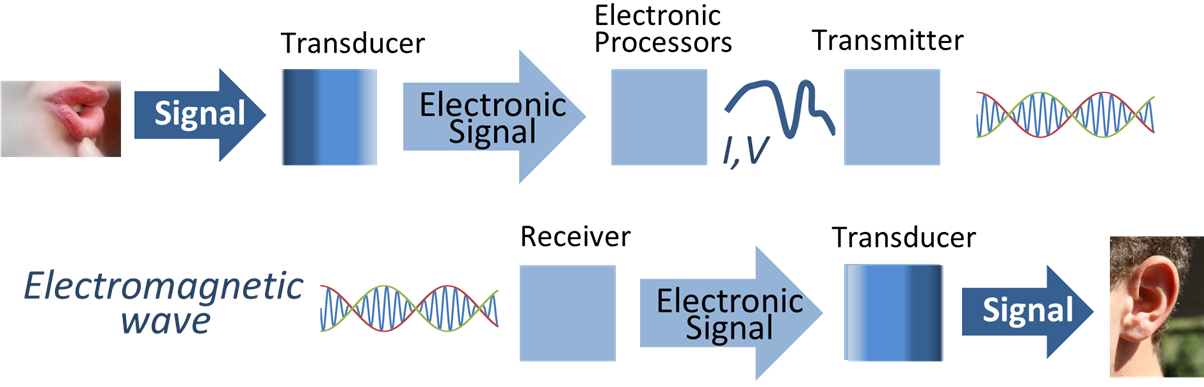
An ion-selective electrode (ISE), also known as a specific ion electrode (SIE), is a simple membrane-based potentiometric device which measures the activity of ions in solution. It is a transducer (or sensor) that converts the change in the concentration of a specific ion dissolved in a solution into an electrical potential. ISE is a type of sensor device that senses changes in signal based on the surrounding environment through time. This device will have an input signal, a property that we wish to quantify, and an output signal, a quantity we can register. In this case, ion selective electrode are electrochemical sensors that give potentiometric signals. The voltage is theoretically dependent on the logarithm of the ionic activity, according to the Nernst equation. Analysis with ISEs expands throughout a range of technological fields such as biology, chemistry, environmental science and other industrial workplaces like agriculture. Ion-selective electrodes are used in analytical ch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transducer
A transducer is a device that Energy transformation, converts energy from one form to another. Usually a transducer converts a signal in one form of energy to a signal in another. Transducers are often employed at the boundaries of automation, Measuring instrument, measurement, and control systems, where electrical signals are converted to and from other physical quantities (energy, force, torque, light, motion, position, etc.). The process of converting one form of energy to another is known as transduction. Types * Mechanical transducers convert physical quantities into mechanical outputs or vice versa; * Electrical transducers convert physical quantities into electrical outputs or signals. Examples of these are: ** a thermocouple that changes temperature differences into a small voltage; ** a linear variable differential transformer (LVDT), used to measure displacement (position) changes by means of electrical signals. Sensors, actuators and transceivers Transducers can be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chalcogenide
: 220px, Cadmium sulfide, a prototypical metal chalcogenide, is used as a yellow pigment. A chalcogenide is a chemical compound consisting of at least one chalcogen anion and at least one more electropositive element. Although all group 16 elements of the periodic table are defined as chalcogens, the term chalcogenide is more commonly reserved for sulfides, selenides, tellurides, and polonides, rather than oxides. Many metal ores exist as chalcogenides. Photoconductive chalcogenide glasses are used in xerography. Some pigments and catalysts are also based on chalcogenides. The metal dichalcogenide MoS2 is a common solid lubricant. Alkali metal and alkaline earth chalcogenides Alkali metal and alkaline earth monochalcogenides are salt-like, being colourless and often water-soluble. The sulfides tend to undergo hydrolysis to form derivatives containing bisulfide (SH−) anions. The alkali metal chalcogenides often crystallize with the antifluorite structure and the alkal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Valinomycin
Valinomycin is a naturally occurring dodecadepsipeptide used in the transport of potassium and as an antibiotic. Valinomycin is obtained from the cells of several ''Streptomyces'' species, '' S. fulvissimus'' being a notable one. It is a member of the group of natural neutral ionophores because it does not have a residual charge. It consists of enantiomers D- and L-valine (Val), D- alpha-hydroxyisovaleric acid, and L-lactic acid. Structures are alternately bound via amide and ester bridges. Valinomycin is highly selective for potassium ions over sodium ions within the cell membrane. It functions as a potassium-specific transporter and facilitates the movement of potassium ions through lipid membranes "down" the electrochemical potential gradient. The stability constant K for the potassium-valinomycin complex is nearly 100,000 times larger than that of the sodium-valinomycin complex. This difference is important for maintaining the selectivity of valinomycin for the transport of p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Selective Electrode
Potassium selective electrodes are a type of ion selective electrode used in biochemical and biophysical research, where measurements of potassium concentration in an aqueous solution are required, usually on a real time basis. These electrodes are typical ion exchange resin membrane electrodes, using valinomycin, a potassium ionophore, as the ion carrier in the membrane to provide the potassium specificity. This type of ion-selective electrode is subject to interference from (in declining order of magnitude) rubidium, caesium, ammonium, sodium, calcium, magnesium, and lithium Lithium (from , , ) is a chemical element; it has chemical symbol, symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard temperature and pressure, standard conditions, it is the least dense metal and the .... The most significant interference with measurement of potassium concentration is from the ammonium ion, which in practice is a problem where the ammonium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compound (chemistry), compounds, produces unique physical property, physical properties including toughness, high rubber elasticity, elasticity, viscoelasticity, and a tendency to form Amorphous solid, amorphous and crystallization of polymers, semicrystalline structures rath ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ion-exchange Resin
An ion-exchange resin or ion-exchange polymer is a resin or polymer that acts as a medium for ion exchange, that is also known as an ionex. It is an insoluble matrix (or support structure) normally in the form of small (0.25–1.43 mm radius) microbeads, usually white or yellowish, fabricated from an organic polymer substrate. The beads are typically porous (with a specific size distribution that will affect its properties), providing a large surface area on and inside them where the trapping of ions occurs along with the accompanying release of other ions, and thus the process is called ion exchange. There are multiple types of ion-exchange resin, that differ in composition if the target is an anion or a cation and are created based on the task they are required for. Most commercial resins are made of polystyrene sulfonateFrançois Dardel and Thomas V. Arden "Ion Exchangers" in Ullmann's Encyclopedia of Industrial Chemistry, 2008, Wiley-VCH, Weinheim. . which is followe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
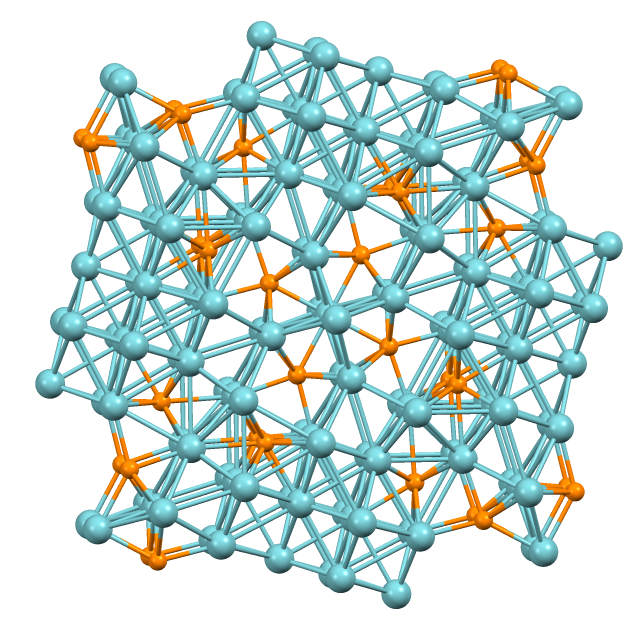
Lanthanum Trifluoride
Lanthanum trifluoride is a refractory ionic compound of lanthanum and fluorine. The chemical formula is . The LaF3 structure Bonding is ionic with lanthanum highly coordinated. The cation sits at the center of a trigonal prism. Nine fluorine atoms are close: three at the bottom corners of the trigonal prism, three in the faces of the trigonal prism, and three at top corners of the trigonal prism. There are also two fluorides a little further away above and below the prism. The cation can be considered 9-coordinate or 11-coordinate. At 300 K, the structure allows the formation of Schottky defects with an activation energy of 0.07 eV, and free flow of fluoride ions with an activation energy of 0.45 eV, making the crystal unusually electrically conductive. The larger sized rare earth elements (lanthanides), which are those with smaller atomic number, also form trifluorides with the LaF3 structure. Some actinides do as well. Applications This white salt is som ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluoride Selective Electrode
A fluoride selective electrode is a type of ion selective electrode sensitive to the concentration of the fluoride ion. A common example is the lanthanum fluoride electrode. Lanthanum fluoride electrode In the lanthanum fluoride electrode, the sensing element is a crystal of lanthanum fluoride (LaF3), doped with europium(II) fluoride (EuF2) to create lattice vacancies. Such a crystal is an ionic conductor by virtue of the mobility of fluoride ions which jump between lattice vacancies. An electrochemical cell may be constructed using such a crystal as a membrane separating two fluoride solutions. This cell acts as a concentration cell with transference where the fluoride transport number is 1. As transference of charge through the crystal is almost exclusively due to fluoride, the electrode is highly specific to fluoride. The only ion which significantly interferes is hydroxide (OH−). Generally such "alkaline error" can be avoided by buffering the sample to a pH below 7. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons (e.g. K+ ( potassium ion)) while an anion is a negatively charged ion with more electrons than protons (e.g. Cl− ( chloride ion) and OH− ( hydroxide ion)). Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed ''monatomic ions'', ''atomic ions'' or ''simple ions'', while ions consisting of two or more atoms are termed polyatomic ions or ''molecular ions''. If only a + or − is present, it indi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or a gas). In electrochemical cells, electrodes are essential parts that can consist of a variety of materials (chemicals) depending on the type of cell. An electrode may be called either a cathode or anode according to the direction of the electric current, unrelated to the potential difference between electrodes. Michael Faraday coined the term "" in 1833; the word recalls the Greek ἤλεκτρον (, "amber") and ὁδός (, "path, way"). The electrophore, invented by Johan Wilcke in 1762, was an early version of an electrode used to study static electricity. Anode and cathode in electrochemical cells Electrodes are an essential part of any battery. The first electrochemical battery was devised by Alessandro Volta and was aptly named the Voltaic cell. This battery consisted of a stack of copper and zinc electrodes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystal Structure
In crystallography, crystal structure is a description of ordered arrangement of atoms, ions, or molecules in a crystalline material. Ordered structures occur from intrinsic nature of constituent particles to form symmetric patterns that repeat along the principal directions of three-dimensional space in matter. The smallest group of particles in a material that constitutes this repeating pattern is the unit cell of the structure. The unit cell completely reflects the symmetry and structure of the entire crystal, which is built up by repetitive translation of the unit cell along its principal axes. The translation vectors define the nodes of the Bravais lattice. The lengths of principal axes/edges, of the unit cell and angles between them are lattice constants, also called ''lattice parameters'' or ''cell parameters''. The symmetry properties of a crystal are described by the concept of space groups. All possible symmetric arrangements of particles in three-dimensional space ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PH Glass Electrode
A glass electrode is a type of ion-selective electrode made of a doped glass membrane that is sensitive to a specific ion. The most common application of ion-selective glass electrodes is for the measurement of pH. The pH electrode is an example of a glass electrode that is sensitive to hydrogen ions. Glass electrodes play an important part in the instrumentation for chemical analysis, and physicochemical studies. The voltage of the glass electrode, relative to some reference value, is sensitive to changes in the activity of certain types of ions. History The first studies of glass electrodes (GE) found different sensitivities of different glasses to change the medium's acidity ( pH), due to the effects of the alkali metal ions. In 1906, M. Cremer, the father of Erika Cremer, determined that the electric potential that arises between parts of the fluid, located on opposite sides of the glass membrane, is proportional to the concentration of acid (hydrogen ion concentration). ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |