|
Inelastic Mean Free Path
The inelastic mean free path (IMFP) is an index of how far an electron on average travels through a solid before losing energy. If a monochromatic, primary beam of electrons is incident on a solid surface, the majority of incident electrons lose their energy because they interact strongly with matter, leading to plasmon excitation, electron-hole pair formation, and vibrational excitation. The intensity of the primary electrons, , is damped as a function of the distance, , into the solid. The intensity decay can be expressed as follows: : I(d) = I_0 \ e^ where is the intensity after the primary electron beam has traveled through the solid to a distance . The parameter , termed the inelastic mean free path (IMFP), is defined as the distance an electron beam can travel before its intensity decays to of its initial value. (Note that this is equation is closely related to the Beer–Lambert law.) The inelastic mean free path of electrons can roughly be described by a univers ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up quark, up and down quark, down quarks. Electrons are extremely lightweight particles that orbit the positively charged atomic nucleus, nucleus of atoms. Their negative charge is balanced by the positive charge of protons in the nucleus, giving atoms their overall electric charge#Charge neutrality, neutral charge. Ordinary matter is composed of atoms, each consisting of a positively charged nucleus surrounded by a number of orbiting electrons equal to the number of protons. The configuration and energy levels of these orbiting electrons determine the chemical properties of an atom. Electrons are bound to the nucleus to different degrees. The outermost or valence electron, valence electrons are the least tightly bound and are responsible for th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
E (mathematical Constant)
The number is a mathematical constant approximately equal to 2.71828 that is the base of a logarithm, base of the natural logarithm and exponential function. It is sometimes called Euler's number, after the Swiss mathematician Leonhard Euler, though this can invite confusion with Euler numbers, or with Euler's constant, a different constant typically denoted \gamma. Alternatively, can be called Napier's constant after John Napier. The Swiss mathematician Jacob Bernoulli discovered the constant while studying compound interest. The number is of great importance in mathematics, alongside 0, 1, Pi, , and . All five appear in one formulation of Euler's identity e^+1=0 and play important and recurring roles across mathematics. Like the constant , is Irrational number, irrational, meaning that it cannot be represented as a ratio of integers, and moreover it is Transcendental number, transcendental, meaning that it is not a root of any non-zero polynomial with rational coefficie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of every atom of that element. The atomic number can be used to uniquely identify ordinary chemical elements. In an ordinary uncharged atom, the atomic number is also equal to the number of electrons. For an ordinary atom which contains protons, neutrons and electrons, the sum of the atomic number ''Z'' and the neutron number ''N'' gives the atom's atomic mass number ''A''. Since protons and neutrons have approximately the same mass (and the mass of the electrons is negligible for many purposes) and the mass defect of the nucleon binding is always small compared to the nucleon mass, the atomic mass of any atom, when expressed in daltons (making a quantity called the " relative isotopic mass"), is within 1% ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transmission Electron Microscope
Transmission electron microscopy (TEM) is a microscopy technique in which a beam of electrons is transmitted through a specimen to form an image. The specimen is most often an ultrathin section less than 100 nm thick or a suspension on a grid. An image is formed from the interaction of the electrons with the sample as the beam is transmitted through the specimen. The image is then magnified and focused onto an imaging device, such as a fluorescent screen, a layer of photographic film, or a detector such as a scintillator attached to a charge-coupled device or a direct electron detector. Transmission electron microscopes are capable of imaging at a significantly higher resolution than light microscopes, owing to the smaller de Broglie wavelength of electrons. This enables the instrument to capture fine detail—even as small as a single column of atoms, which is thousands of times smaller than a resolvable object seen in a light microscope. Transmission electron micro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Energy Loss Spectroscopy
Electron energy loss spectroscopy (EELS) is a form of electron microscopy in which a material is exposed to a beam of electrons with a known, narrow range of kinetic energies. Some of the electrons will undergo inelastic scattering, which means that they lose energy and have their paths slightly and randomly deflected. The amount of energy loss can be measured via an electron spectrometer and interpreted in terms of what caused the energy loss. Inelastic interactions include phonon excitations, inter- and intra- band transitions, plasmon excitations, inner shell ionizations, and Cherenkov radiation. The inner-shell ionizations are particularly useful for detecting the elemental components of a material. For example, one might find that a larger-than-expected number of electrons comes through the material with 285 eV less energy than they had when they entered the material. This is approximately the amount of energy needed to remove an inner-shell electron from a c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Microscope
An electron microscope is a microscope that uses a beam of electrons as a source of illumination. It uses electron optics that are analogous to the glass lenses of an optical light microscope to control the electron beam, for instance focusing it to produce magnified images or electron diffraction patterns. As the wavelength of an electron can be up to 100,000 times smaller than that of visible light, electron microscopes have a much higher Angular resolution, resolution of about 0.1 nm, which compares to about 200 nm for optical microscope, light microscopes. ''Electron microscope'' may refer to: * Transmission electron microscopy, Transmission electron microscope (TEM) where swift electrons go through a thin sample * Scanning transmission electron microscopy, Scanning transmission electron microscope (STEM) which is similar to TEM with a scanned electron probe * Scanning electron microscope (SEM) which is similar to STEM, but with thick samples * Electron microprobe sim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
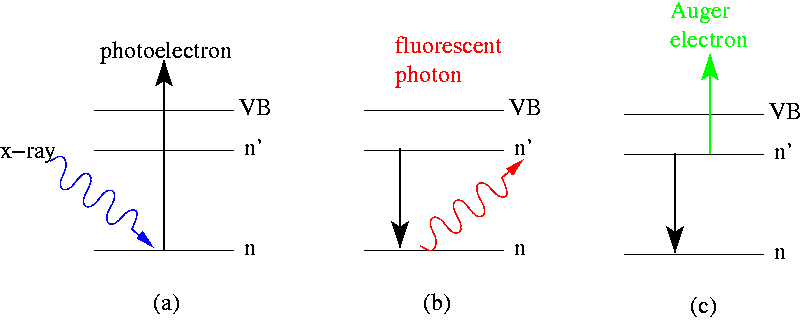
Electron Spectroscopy
Electron spectroscopy refers to a group formed by techniques based on the analysis of the energies of emitted electrons such as Photoelectric effect, photoelectrons and Auger electrons. This group includes X-ray photoelectron spectroscopy (XPS), which also known as Electron Spectroscopy for Chemical Analysis (ESCA), Electron energy loss spectroscopy (EELS), Ultraviolet photoelectron spectroscopy (UPS), and Auger electron spectroscopy (AES). These analytical techniques are used to identify and determine the elements and their electronic structures from the surface of a test sample. Samples can be solids, gases or liquids.Yang Leng; ''Materials Characterization: Introduction to Microscopic and Spectroscopic Methods (Second Edition)''; Publisher John Wiley & Sons, Incorporated 2013; p: 191-192, 221-224.Daintith, J.; ''Dictionary of Chemistry (6th Edition)''; Oxford University Press, 2008; p: 191, 416, 541 Chemical information is obtained only from the uppermost atomic layers of the sam ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beer–Lambert Law
The Beer–Bouguer–Lambert (BBL) extinction law is an empirical relationship describing the attenuation in intensity of a radiation beam passing through a macroscopically homogenous medium with which it interacts. Formally, it states that the intensity of radiation decays exponentially in the absorbance of the medium, and that said absorbance is proportional to the length of beam passing through the medium, the concentration of interacting matter along that path, and a constant representing said matter's propensity to interact. The extinction law's primary application is in chemical analysis, where it underlies the Beer–Lambert law, commonly called Beer's law. Beer's law states that a beam of visible light passing through a chemical solution of fixed geometry experiences absorption proportional to the solute concentration. Other applications appear in physical optics, where it quantifies astronomical extinction and the absorption of photons, neutrons, or rarefied gas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Damped
In physical systems, damping is the loss of energy of an oscillating system by dissipation. Damping is an influence within or upon an oscillatory system that has the effect of reducing or preventing its oscillation. Examples of damping include viscous damping in a fluid (see viscous drag), surface friction, radiation, resistance in electronic oscillators, and absorption and scattering of light in optical oscillators. Damping not based on energy loss can be important in other oscillating systems such as those that occur in biological systems and bikes (ex. Suspension (mechanics)). Damping is not to be confused with friction, which is a type of dissipative force acting on a system. Friction can cause or be a factor of damping. Many systems exhibit oscillatory behavior when they are disturbed from their position of static equilibrium. A mass suspended from a spring, for example, might, if pulled and released, bounce up and down. On each bounce, the system tends to return to i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solid
Solid is a state of matter where molecules are closely packed and can not slide past each other. Solids resist compression, expansion, or external forces that would alter its shape, with the degree to which they are resisted dependent upon the specific material under consideration. Solids also always possess the least amount of kinetic energy per atom/molecule relative to other phases or, equivalently stated, solids are formed when matter in the liquid / gas phase is cooled below a certain temperature. This temperature is called the melting point of that substance and is an intrinsic property, i.e. independent of how much of the matter there is. All matter in solids can be arranged on a microscopic scale under certain conditions. Solids are characterized by structural rigidity and resistance to applied external forces and pressure. Unlike liquids, solids do not flow to take on the shape of their container, nor do they expand to fill the entire available volume like a gas. Much ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intensity (physics)
In physics and many other areas of science and engineering the intensity or flux of radiant energy is the Power (physics), power transferred per unit area, where the area is measured on the plane perpendicular to the direction of propagation of the energy. In the SI system, it has units watts per square metre (W/m2), or kilogram, kg⋅second, s−3 in SI base unit, base units. Intensity is used most frequently with waves such as acoustic waves (sound), matter waves such as electrons in electron microscopes, and electromagnetic waves such as light or radio waves, in which case the time averaging, ''average'' power transfer over one Period (physics), period of the wave is used. ''Intensity'' can be applied to other circumstances where energy is transferred. For example, one could calculate the intensity of the kinetic energy carried by drops of water from a garden sprinkler. The word "intensity" as used here is not synonymous with "wikt:strength, strength", "wikt:amplitude, amplitude ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Hole
In physics, chemistry, and electronic engineering, an electron hole (often simply called a hole) is a quasiparticle denoting the lack of an electron at a position where one could exist in an atom or crystal structure, atomic lattice. Since in a normal atom or crystal lattice the negative charge of the electrons is balanced by the positive charge of the atomic nucleus, atomic nuclei, the absence of an electron leaves a net positive charge at the hole's location. Holes in a metal or semiconductor crystal lattice can move through the lattice as electrons can, and act similarly to electric charge, positively-charged particles. They play an important role in the operation of semiconductor devices such as transistors, diodes (including light-emitting diodes) and integrated circuits. If an electron is excited into a higher state it leaves a hole in its old state. This meaning is used in Auger electron spectroscopy (and other x-ray techniques), in computational chemistry, and to explai ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |