|
Immunolabeling
Immunolabeling is a biochemical process that enables the detection and localization of an antigen to a particular site within a cell, tissue, or organ. Antigens are organic molecules, usually proteins, capable of binding to an antibody. These antigens can be visualized using a combination of antigen-specific antibody as well as a means of detection, called a tag, that is covalently linked to the antibody. If the immunolabeling process is meant to reveal information about a cell or its substructures, the process is called immunocytochemistry. Immunolabeling of larger structures is called immunohistochemistry. There are two complex steps in the manufacture of antibody for immunolabeling. The first is producing the antibody that binds specifically to the antigen of interest and the second is fusing the tag to the antibody. Since it is impractical to fuse a tag to every conceivable antigen-specific antibody, most immunolabeling processes use an indirect method of detection. Thi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Primary And Secondary Antibodies
Primary and secondary antibodies are two groups of antibodies that are classified based on whether they bind to ''antigens or proteins'' directly or target another (primary) antibody that, in turn, is bound to an ''antigen or protein''. Primary A primary antibody can be very useful for the detection of biomarkers for diseases such as cancer, diabetes, Parkinson’s and Alzheimer’s disease and they are used for the study of absorption, distribution, metabolism, and excretion (ADME) and multi-drug resistance (MDR) of therapeutic agents. Secondary Secondary antibodies provide signal detection and amplification along with extending the utility of an antibody through conjugation to proteins. Secondary antibodies are especially efficient in immunolabeling. Secondary antibodies bind to primary antibodies, which are directly bound to the target antigen(s). In immunolabeling, the primary antibody's Fab domain binds to an antigen and exposes its Fc domain to secondary antibody. Then ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Analytical Biochemistry
''Analytical Biochemistry'' is a peer-reviewed scientific journal established in 1960. It covers the field of biochemistry. According to the ''Journal Citation Reports'', the journal has a 2014 impact factor of 2.219. Abstracting and Indexing The journal is abstracted and indexed in Analytical Abstracts, Biological Abstracts, Chemical Abstracts, Current Contents/Life Sciences, EMBASE, EMBiology, MEDLINE, Science Citation Index, and Scopus Scopus is Elsevier's abstract and citation database launched in 2004. Scopus covers nearly 36,377 titles (22,794 active titles and 13,583 inactive titles) from approximately 11,678 publishers, of which 34,346 are peer-reviewed journals in top-l .... References External links * Publications established in 1960 Biochemistry journals Elsevier academic journals English-language journals Biweekly journals {{biochem-journal-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Affinity Chromatography
Affinity chromatography is a method of separating a biomolecule from a mixture, based on a highly specific macromolecular binding interaction between the biomolecule and another substance. The specific type of binding interaction depends on the biomolecule of interest; antigen and antibody, enzyme and substrate, receptor and ligand, or protein and nucleic acid binding interactions are frequently exploited for isolation of various biomolecules. Affinity chromatography is useful for its high selectivity and resolution of separation, compared to other chromatographic methods. Principle Affinity chromatography has the advantage of specific binding interactions between the analyte of interest (normally dissolved in the mobile phase), and a binding partner or ligand (immobilized on the stationary phase). In a typical affinity chromatography experiment, the ligand is attached to a solid, insoluble matrix—usually a polymer such as agarose or polyacrylamide—chemically m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyclonal Antibodies
Polyclonal antibodies (pAbs) are antibodies that are secreted by different B cell lineages within the body (whereas monoclonal antibodies come from a single cell lineage). They are a collection of immunoglobulin molecules that react against a specific antigen, each identifying a different epitope. Production The general procedure to produce polyclonal antibodies is as follows: # Antigen preparation # Adjuvant selection and preparation # Animal selection # Injection process # Blood serum extraction An antigen/adjuvant conjugate is injected into an animal of choice to initiate an amplified immune response. After a series of injections over a specific length of time, the animal is expected to have created antibodies against the conjugate. Blood is then extracted from the animal and then purified to obtain the antibody of interest. Inoculation is performed on a suitable mammal, such as a mouse, rabbit or goat. Larger mammals are often preferred as the amount of serum that can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antigens
In immunology, an antigen (Ag) is a molecule or molecular structure or any foreign particulate matter or a pollen grain that can bind to a specific antibody or T-cell receptor. The presence of antigens in the body may trigger an immune response. The term ''antigen'' originally referred to a substance that is an antibody generator. Antigens can be proteins, peptides (amino acid chains), polysaccharides (chains of monosaccharides/simple sugars), lipids, or nucleic acids. Antigens are recognized by antigen receptors, including antibodies and T-cell receptors. Diverse antigen receptors are made by cells of the immune system so that each cell has a specificity for a single antigen. Upon exposure to an antigen, only the lymphocytes that recognize that antigen are activated and expanded, a process known as clonal selection. In most cases, an antibody can only react to and bind one specific antigen; in some instances, however, antibodies may cross-react and bind more than one antigen. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotype (immunology)
In immunology, antibodies ( immunoglobulins (Ig)) are classified into several types called isotypes or classes. The variable (V) regions near the tip of the antibody can differ from molecule to molecule in countless ways, allowing it to specifically target an antigen (or more exactly, an epitope). In contrast, the constant (C) regions only occur in a few variants, which define the antibody's class. Antibodies of different classes activate distinct effector mechanisms in response to an antigen (triggering different elements of the innate immune system). They appear at different stages of an immune response, differ in structural features, and in their location around the body. Isotype expression reflects the maturation stage of a B cell. Naive B cells express IgM and IgD isotypes with unmutated variable genes, which are produced from the same initial transcript following alternative splicing. Expression of other antibody isotypes (in humans: IgG, IgA, and IgE) occurs via a process ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoclonal Antibodies
A monoclonal antibody (mAb, more rarely called moAb) is an antibody produced from a cell Lineage made by cloning a unique white blood cell. All subsequent antibodies derived this way trace back to a unique parent cell. Monoclonal antibodies can have monovalent affinity, binding only to the same epitope (the part of an antigen that is recognized by the antibody). In contrast, polyclonal antibodies bind to multiple epitopes and are usually made by several different antibody-secreting plasma cell lineages. Bispecific monoclonal antibodies can also be engineered, by increasing the therapeutic targets of one monoclonal antibody to two epitopes. It is possible to produce monoclonal antibodies that specifically bind to virtually any suitable substance; they can then serve to detect or purify it. This capability has become an investigative tool in biochemistry, molecular biology, and medicine. Monoclonal antibodies are being used on a clinical level for both the diagnosis and therap ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
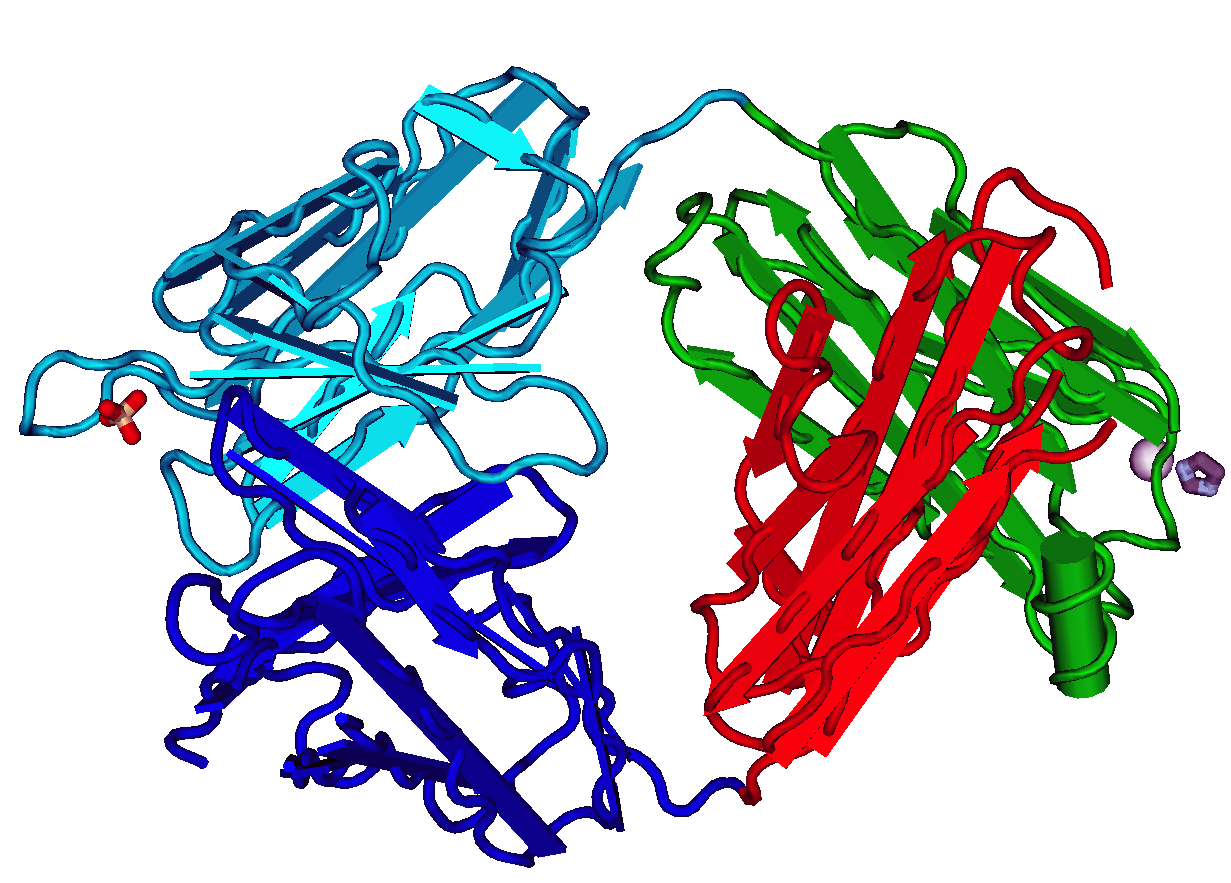
Fragment Antigen-binding
The fragment antigen-binding region (Fab region) is a region on an antibody that binds to antigens. It is composed of one constant and one variable domain of each of the heavy and the light chain. The variable domain contains the paratope (the antigen-binding site), comprising a set of complementarity-determining regions, at the amino terminal end of the monomer. Each arm of the Y thus binds an epitope on the antigen. Preparation In an experimental setting, Fc and Fab fragments can be generated in the laboratory. The enzyme papain can be used to cleave an immunoglobulin monomer into two Fab fragments and an Fc fragment. Conversely, the enzyme pepsin cleaves below the hinge region, so the result instead is a F(ab')2 fragment and a pFc' fragment. Recently another enzyme for generation of F(ab')2 has been commercially available. The enzyme IdeS (Immunoglobulin degrading enzyme from ''Streptococcus pyogenes'', trade name FabRICATOR) cleaves IgG in a sequence specific manner at ne ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fragment Crystallizable Region
The fragment crystallizable region (Fc region) is the tail region of an antibody that interacts with cell surface receptors called Fc receptors and some proteins of the complement system. This property allows antibodies to activate the immune system. In IgG, IgA and IgD antibody isotypes, the Fc region is composed of two identical protein fragments, derived from the second and third constant domains of the antibody's two heavy chains; IgM and IgE Fc regions contain three heavy chain constant domains (CH domains 2–4) in each polypeptide chain. The Fc regions of IgGs bear a highly conserved N-glycosylation site. Glycosylation of the Fc fragment is essential for Fc receptor-mediated activity. The N-glycans attached to this site are predominantly core- fucosylated diantennary structures of the complex type. In addition, small amounts of these N-glycans also bear bisecting GlcNAc and α-2,6 linked sialic acid residues. The other part of an antibody, called the Fab regio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Immunoglobulin G
Immunoglobulin G (Ig G) is a type of antibody. Representing approximately 75% of serum antibodies in humans, IgG is the most common type of antibody found in blood circulation. IgG molecules are created and released by plasma B cells. Each IgG antibody has two paratopes. Function Antibodies are major components of humoral immunity. IgG is the main type of antibody found in blood and extracellular fluid, allowing it to control infection of body tissues. By binding many kinds of pathogens such as viruses, bacteria, and fungi, IgG protects the body from infection. It does this through several mechanisms: * IgG-mediated binding of pathogens causes their immobilization and binding together via agglutination; IgG coating of pathogen surfaces (known as opsonization) allows their recognition and ingestion by phagocytic immune cells leading to the elimination of the pathogen itself; * IgG activates all the classical pathway of the complement system, a cascade of immune prote ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |